Abstract
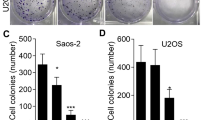
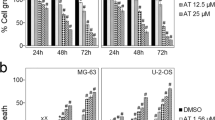
Dihydroartemisinin (DHA) exhibits antitumor activity against a wide spectrum of cancer cells. However, whether DHA has anti-tumor effect on human osteosarcoma cells remains unknown. This study aims to investigate the anti-tumor activity of DHA and the underlying mechanisms in human osteosarcoma cell lines with different p53 mutation statuses. Four human osteosarcoma cell lines were treated with different concentrations of DHA. Then, cell proliferation was determined by the CCK-8 viability assay; apoptosis and cell cycle progression were evaluated by flow cytometry; protein expression was analyzed by western blot assay; and NF-kB activity was examined by luciferase assay. The results demonstrated that DHA treatment could inhibit the proliferation of four osteosarcoma cell lines in a dose-dependent manner. P53 wild-type osteosarcoma cells were more sensitive to DHA. Moreover, the percentage of apoptotic cell and cell arrest in G2/M phase was increased upon DHA treatment in a dose-dependent manner. Mechanistically, DHA activated caspase-3, caspase-8, and caspase-9; upregulated the expression of Bax, FAS, and cyclin D1; downregulated the expression of Bcl-2, Cdc25B, and cyclin B1; and inhibited the activity of NF-кB. In conclusion, DHA has significant anticancer effects against human osteosarcoma cells, which include induction of apoptosis and cell cycle arrest. The p53 gene may play a certain role in the DHA-induced human osteosarcoma apoptosis and cell cycle arrest. DHA is a novel anti-osteosarcoma drug candidate that merits further study.





Similar content being viewed by others
Abbreviations
- DHA:
-
Dihydroartemisinin
- DMSO:
-
Dimethyl sulfoxide
- MEM:
-
Modified eagle medium
- FBS:
-
Fetal bovine serum
- BCA:
-
Bicinchoninic acid
- CCK-8:
-
Cell counting kit-8
- ATCC:
-
American type culture collection
- PS:
-
Phosphatidylserine
- TNF:
-
Tumor necrosis factor
- FASL:
-
Fas ligand
- FADD:
-
Fas-associated protein with death domain
- NF-кB:
-
Nuclear factor-kappa b
References
Tan JZ, Schlicht SM, Powell GJ, Thomas D, Slavin JL, Smith PJ, Choong PF (2006) Multidisciplinary approach to diagnosis and management of osteosarcoma: a review of the St Vincent’s hospital experience. Int Semin Surg Oncol 3:38. doi:10.1186/1477-7800-3-38
Ferrari S, Palmerini E (2007) Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol 19(4):341–346. doi:10.1097/CCO.0b013e328122d73f
Marcove RC, Mike V, Hajek JV, Levin AG, Hutter RV (1970) Osteogenic sarcoma under the age of twenty-one: a review of one hundred and forty-five operative cases. J Bone Joint Surg Am 52(3):411–423
Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B, Urban C (1982) Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 49(6):1221–1230
Kager L, Zoubek A, Kastner U, Kempf-Bielack B, Potratz J, Kotz R, Exner GU, Franzius C, Lang S, Maas R, Jurgens H, Gadner H, Bielack S (2006) Skip metastases in osteosarcoma: experience of the cooperative osteosarcoma study group. J Clin Oncol 24(10):1535–1541. doi:10.1200/JCO.2005.04.2978
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from china. Science 228(4703):1049–1055. doi:10.1126/science.3887571
O’Neill PM, Posner GH (2004) A medicinal chemistry perspective on artemisinin and related endoperoxides. J Med Chem 47(12):2945–2964. doi:10.1021/jm030571c
Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim HJ, Cha IH (2007) Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck 29(4):335–340. doi:10.1002/hed.20524
Chen HH, Zhou HJ, Fang X (2003) Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res 48(3):231–236. doi:10.1016/S1043-6618(03)00107-5
Singh NP, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci 70(1):49–56. doi:10.1016/S0024-3205(01)01372-8
Efferth T, Olbrich A, Bauer R (2002) mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochem Pharmacol 64(4):617–623. doi:10.1016/S0006-2952(02)01221-2
Moore JC, Lai H, Li JR, Ren RL, McDougall JA, Singh NP, Chou CK (1995) Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett 98(1):83–87. doi:10.1016/S0304-3835(06)80014-5
Lai H, Singh NP (1995) Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett 91(1):41–46. doi:10.1016/0304-3835(94)03716-V
Lai H, Singh NP (2006) Oral artemisinin prevents and delays the development of 7, 12-dimethylbenz[a]anthracene (dmba)-induced breast cancer in the rat. Cancer Lett 231(1):43–48. doi:10.1016/j.canlet.2005.01.019
Dell’Eva R, Pfeffer U, Vene R, Anfosso L, Forlani A, Albini A, Efferth T (2004) Inhibition of angiogenesis in vivo and growth of kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochem Pharmacol 68(12):2359–2366. doi:10.1016/j.bcp.2004.08.021
Disbrow GL, Baege AC, Kierpiec KA, Yuan H, Centeno JA, Thibodeaux CA, Hartmann D, Schlegel R (2005) Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res 65(23):10854–10861. doi:10.1158/0008-5472.CAN-05-1216
Singh NP, Lai HC (2004) Artemisinin induces apoptosis in human cancer cells. Anticancer Res 24(4):2277–2280
Lee J, Zhou HJ, Wu XH (2006) Dihydroartemisinin downregulates vascular endothelial growth factor expression and induces apoptosis in chronic myeloid leukemia k562 cells. Cancer Chemother Pharmacol 57(2):213–220. doi:10.1007/s00280-005-0002-y
Longo M, Zanoncelli S, Manera D, Brughera M, Colombo P, Lansen J, Mazue G, Gomes M, Taylor WR, Olliaro P (2006) Effects of the antimalarial drug dihydroartemisinin (dha) on rat embryos in vitro. Reprod Toxicol 21(1):83–93. doi:10.1016/j.reprotox.2005.05.005
Wu XH, Zhou HJ, Lee J (2006) Dihydroartemisinin inhibits angiogenesis induced by multiple myeloma rpmi8226 cells under hypoxic conditions via downregulation of vascular endothelial growth factor expression and suppression of vascular endothelial growth factor secretion. Anticancer Drugs 17(7):839–848. doi:10.1097/01.cad.0000224443.85834.32
Jung M, Tak J, Chung WY, Park KK (2006) Antiangiogenic activity of deoxoartemisinin derivatives on chorioallantoic membrane. Bioorg Med Chem Lett 16(5):1227–1230. doi:10.1016/j.bmcl.2005.11.074
Efferth T (2006) Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr Drug Targets 7(4):407–421. doi:10.2174/138945006776359412
Jung M, Lee S, Ham J, Lee K, Kim H, Kim SK (2003) Antitumor activity of novel deoxoartemisinin monomers, dimers, and trimer. J Med Chem 46(6):987–994. doi:10.1021/jm020119d
Chandar N, Billig B, McMaster J, Novak J (1992) Inactivation of p53 gene in human and murine osteosarcoma cells. Br J Cancer 65(2):208–214. doi:10.1038/bjc
Hellwinkel OJ, Müller J, Pollmann A, Kabisch H (2005) Osteosarcoma cell lines display variable individual reactions on wildtype p53 and Rb tumour-suppressor transgenes. J Gene Med 7(4):407–419. doi:10.1002/jgm.684
Konopleva M, Zhao S, Xie Z, Segall H, Younes A, Claxton DF, Estrov Z, Kornblau SM, Andreeff M (1999) Apoptosis: molecules and mechanisms. Adv Exp Med Biol 457:217–236
Staudt LM (2010) Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol 2(6):a000109
Takeshita H, Gebhardt MC, Springfield DS, Kusuzaki K, Mankin HJ (1996) Experimental models for the study of drug resistance in osteosarcoma: P-glycoprotein-positive, murine osteosarcoma cell lines. J Bone Joint Surg Am 78(3):366–375
Nakase I, Lai H, Singh NP, Sasaki T (2008) Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm 354(1–2):28–33. doi:10.1016/j.ijpharm.2007.09.003
Veerasubramanian P, Gosi P, Limsomwong C, Walsh DS (2006) Artesunate and a major metabolite, dihydroartemisinin, diminish mitogen-induced lymphocyte proliferation and activation. Southeast Asian J Trop Med Public Health 37(5):838–847
Huang XJ, Ma ZQ, Zhang WP, Lu YB, Wei EQ (2007) Dihydroartemisinin exerts cytotoxic effects and inhibits hypoxia inducible factor-1alpha activation in c6 glioma cells. J Pharm Pharmacol 59(6):849–856. doi:10.1211/jpp.59.6.0011
Jiao Y, Ge CM, Meng QH, Cao JP, Tong J, Fan SJ (2007) Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol Sin 28(7):1045–1056. doi:10.1111/j.1745-7254.2007.00612.x
Mercer AE, Maggs JL, Sun XM, Cohen GM, Chadwick J, O’Neill PM, Park BK (2007) Evidence for the involvement of carbon-centered radicals in the induction of apoptotic cell death by artemisinin compounds. J Biol Chem 282(13):9372–9382. doi:10.1074/jbc.M610375200
Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM (2006) Mitochondria as the target of the pro-apoptotic protein bax. Biochim Biophys Acta 1757(9–10):1301–1311. doi:10.1016/j.bbabio.2006.05.032
Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB (2000) Bcl-2 inhibits bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ 7(1):102–111. doi:10.1038/sj.cdd.4400597
Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y (1998) Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci USA 95(25):14681–14686
Guicciardi ME, Gores GJ (2009) Life and death by death receptors. FASEB J 23(6):1625–1637. doi:10.1096/fj.08-111005
Hartwell LH, Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246(4930):629–634. doi:10.1126/science.2683079
Lindqvist A, Kallstrom H, Karlsson Rosenthal C (2004) Characterisation of cdc25b localisation and nuclear export during the cell cycle and in response to stress. J Cell Sci 117(Pt 21):4979–4990. doi:10.1242/jcs.01395
Donzelli M, Draetta GF (2003) Regulating mammalian checkpoints through cdc25 inactivation. EMBO Rep 4(7):671–677. doi:10.1038/sj.embor.embor887
Taylor WR, Stark GR (2001) Regulation of the g2/m transition by p53. Oncogene 20(15):1803–1815. doi:10.1038/sj.onc.1204252
Haupt S, Berger M, Goldberg Z, Haupt Y (2003) Apoptosis–the p53 network. J Cell Sci 116(Pt 20):4077–4085. doi:10.1242/jcs.00739
Acknowledgments
The authors appreciate the suggestions of Dr. Hua Chen, the First Affiliated Hospital of Harbin Medical University. The authors also thank the anonymous reviewers for their insightful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ye Ji and Yi-Cai Zhang contributed equally.
Rights and permissions
About this article
Cite this article
Ji, Y., Zhang, YC., Pei, LB. et al. Anti-tumor effects of dihydroartemisinin on human osteosarcoma. Mol Cell Biochem 351, 99–108 (2011). https://doi.org/10.1007/s11010-011-0716-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0716-6