Abstract
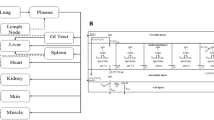
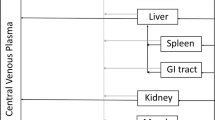
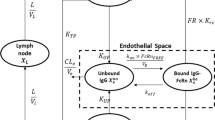
The objectives of the following investigation were (1) development of a physiologically based pharmacokinetic (PBPK) model capable of characterizing the plasma and tissue pharmacokinetics (PK) of nonspecific or antigen specific monoclonal antibodies (mAbs) in wild type, FcRn knockout, tumor bearing and non tumor bearing mice and (2) evaluation of the scale up potential of the model by characterizing the mouse, rat, monkey and human plasma PK of mAbs, simultaneously. A PBPK model containing 15 tissues, a carcass and a tumor compartment was developed by modifying/augmenting previously published PBPK models. Each tissue compartment was subdivided into plasma, blood cell, endothelial, interstitial and cellular sub-compartments. Each tissue was connected through blood and lymph flow to the systemic circulation. Lymph flow was set to a value 500 times lower than plasma flow and vascular reflection coefficients for each tissue were adjusted according to their vascular pore size. In each tissue endothelial space, mAb entered via pinocytosis and the interaction of FcRn with mAb was described by on and off rates. FcRn bound mAb was recycled and unbound mAb was eliminated by a first order process (K deg ). The PBPK model was simultaneously fit to the following datasets to estimate four system parameters: (1) plasma and tissue PK of nonspecific mAb in wild type mouse with or without simultaneous intravenous immunoglobulin (IVIG) administration, (2) plasma and tissue PK of nonspecific mAb in FcRn knockout mouse, (3) plasma and tissue PK of nonspecific mAb in tumor bearing mouse, (4) plasma and tissue PK of tumor antigen specific mAb in tumor bearing mouse, and (5) plasma PK of mAb in rat, monkey and human. The model was able to characterize all the datasets reasonably well with a common set of parameters. The estimated value of the four system parameters i.e. FcRn concentration (FcRn), rate of pinocytosis per unit endosomal space (CL up ), K deg and the proportionality constant (C_LNLF) between the rate at which antibody transfers from the lymph node compartment to the blood compartment and the plasma flow of the given species, were found to be 4.98E−05 M (CV% = 11.1), 3.66E−02 l/h/l (%CV = 3.48), 42.9 1/h (%CV = 15.7) and 9.1 (CV% > 50). Thus, a platform PBPK model has been developed that can not only simultaneously characterize mAb disposition data obtained from various previously published mouse PBPK models but is also capable of characterizing mAb disposition in various preclinical species and human.










Similar content being viewed by others
References
Levy G (1966) Kinetics of pharmacologic effects. Clin Pharmacol Ther 7:362–372
Lobo ED, Hansen RJ, Balthasar JP (2004) Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 93:2645–2668
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Mould DR, Sweeney KR (2007) The pharmacokinetics and pharmacodynamics of monoclonal antibodies-mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel 10:84–96
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532
Mahmood I (2009) Pharmacokinetic allometric scaling of antibodies: application to the first-in-human dose estimation. J Pharm Sci 98:3850–3861
Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, Hurh E, Gibbs MA (2011) Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet 50:131–142
De Buck SS, Sinha VK, Fenu LA, Nijsen MJ, Mackie CE, Gilissen RA (2007) Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos 35:1766–1780
Grime KH, Bird J, Ferguson D, Riley RJ (2009) Mechanism-based inhibition of cytochrome P450 enzymes: an evaluation of early decision making in vitro approaches and drug–drug interaction prediction methods. Eur J Pharm Sci 36:175–191
Thygesen P, Macheras P, Van Peer A (2009) Physiologically-based PK/PD modelling of therapeutic macromolecules. Pharm Res 26:2543–2550
Dedrick RL (1973) Animal scale-up. J Pharmacokinet Biopharm 1:435–461
Bischoff KB, Dedrick RL, Zaharko DS, Longstreth JA (1971) Methotrexate pharmacokinetics. J Pharm Sci 60:1128–1133
Baxter LT, Zhu H, Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res 54:1517–1528
Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK (1995) Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res 55:4611–4622
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34:687–709
Urva SR, Yang VC, Balthasar JP (2010) Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci 99:1582–1600
Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F(ab’)2, and Fab’ in mice. Cancer Res 46:3969–3978
Ferl GZ, Wu AM, DiStefano JJ 3rd (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33:1640–1652
Davda JP, Jain M, Batra SK, Gwilt PR, Robinson DH (2008) A physiologically based pharmacokinetic (PBPK) model to characterize and predict the disposition of monoclonal antibody CC49 and its single chain Fv constructs. Int Immunopharmacol 8:401–413
Garg A (2007). Investigation of the role of FcRn in the absorption, distribution, and elimination of monoclonal antibodies, Chap. 3. PhD Thesis, Department of Pharmaceutical Sciences. 71–111
Williams LE, Wu AM, Yazaki PJ, Liu A, Raubitschek AA, Shively JE, Wong JY (2001) Numerical selection of optimal tumor imaging agents with application to engineered antibodies. Cancer Biother Radiopharm 16:25–35
Bazin-Redureau MI, Renard CB, Scherrmann JM (1997) Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab’)2 and Fab after intravenous administration in the rat. J Pharm Pharmacol 49:277–281
Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, Keller S, Tang MT, Tso JY, Vasquez M, Tsurushita N (2004) Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem 279:6213–6216
Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, Teoh LS, Velagapudi RB, Noertersheuser PA, Granneman GR, Fischkoff SA, Chartash EK (2003) Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther 25:1700–1721
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Swartz MA (2001) The physiology of the lymphatic system. Adv Drug Deliv Rev 50:3–20
Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2:14
Vaughn DE, Bjorkman PJ (1997) High-affinity binding of the neonatal Fc receptor to its IgG ligand requires receptor immobilization. Biochemistry 36:9374–9380
Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N (2006) An engineered human IgG1 antibody with longer serum half-life. J Immunol 176:346–356
Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Jiang W, Wroblewski VJ (2007) Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: relationship to pharmacokinetics in mice and primates. Drug Metab Dispos 35:86–94
Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Wroblewski VJ (2007) Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J Biol Chem 282:1709–1717
Chen Y, Balthasar JP (2010) A physiologically-based pharmacokinetic (PBPK) model for disposition of IgG with altered FcRn binding kinetics, AAPS Annual Meeting, New Orleans, p R6397
Emond C, Birnbaum LS, DeVito MJ (2006) Use of a physiologically based pharmacokinetic model for rats to study the influence of body fat mass and induction of CYP1A2 on the pharmacokinetics of TCDD. Environ Health Perspect 114:1394–1400
Dedrick RL, Bischoff KB (1980) Species similarities in pharmacokinetics. Fed Proc. 39:54–59
Johnson TN (2005) Modelling approaches to dose estimation in children. Br J Clin Pharmacol 59:663–669
Parrott N, Lave T (2002) Prediction of intestinal absorption: comparative assessment of GASTROPLUS and IDEA. Eur J Pharm Sci 17:51–61
Bischoff KB, Dedrick RL (1968) Thiopental pharmacokinetics. J Pharm Sci 57:1346–1351
Roopenian DC, Akilesh S (2007) FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7:715–725
Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES (1996) Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol 26:690–696
Junghans RP, Anderson CL (1996) The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA 93:5512–5516
Brambell FW (1969) The transmission of immune globulins from the mother to the foetal and newborn young. Proc Nutr Soc 28:35–41
Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES (2004) Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol 172:2021–2029
Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES (2009) Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci USA 106:2788–2793
Zhou J, Johnson JE, Ghetie V, Ober RJ, Ward ES (2003) Generation of mutated variants of the human form of the MHC class I-related receptor, FcRn, with increased affinity for mouse immunoglobulin G. J Mol Biol 332:901–913
Haymann JP, Levraud JP, Bouet S, Kappes V, Hagege J, Nguyen G, Xu Y, Rondeau E, Sraer JD (2000) Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 11:632–639
Kobayashi N, Suzuki Y, Tsuge T, Okumura K, Ra C, Tomino Y (2002) FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol 282:F358–F365
Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES (1998) Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 10:1289–1298
Cauza K, Hinterhuber G, Dingelmaier-Hovorka R, Brugger K, Klosner G, Horvat R, Wolff K, Foedinger D (2005) Expression of FcRn, the MHC class I-related receptor for IgG, in human keratinocytes. J Invest Dermatol 124:132–139
Blumberg RS, Koss T, Story CM, Barisani D, Polischuk J, Lipin A, Pablo L, Green R, Simister NE (1995) A major histocompatibility complex class I-related Fc receptor for IgG on rat hepatocytes. J Clin Invest 95:2397–2402
Cianga P, Cianga C, Cozma L, Ward ES, Carasevici E (2003) The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol 64:1152–1159
Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, Lencer WI, Blumberg RS (2001) MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol 166:3266–3276
Akilesh S, Christianson GJ, Roopenian DC, Shaw AS (2007) Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 179:4580–4588
Kandil E, Egashira M, Miyoshi O, Niikawa N, Ishibashi T, Kasahara M (1996) The human gene encoding the heavy chain of the major histocompatibility complex class I-like Fc receptor (FCGRT) maps to 19q13.3. Cytogenet Cell Genet 73:97–98
Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB (2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 101:6062–6067
Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE (1997) Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology 92:69–74
Haigler HT, McKanna JA, Cohen S (1979) Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J Cell Biol 83:82–90
Pease LF III, Elliott JT, Tsai DH, Zachariah MR, Tarlov MJ (2008) Determination of protein aggregation with differential mobility analysis: application to IgG antibody. Biotechnol Bioeng 101:1214–1222
Vugmeyster Y, DeFranco D, Szklut P, Wang Q, Xu X (2010) Biodistribution of [125I]-labeled therapeutic proteins: application in protein drug development beyond oncology. J Pharm Sci 99:1028–1045
Alley SC, Okeley NM, Senter PD (2010) Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol 14:529–537
Doppalapudi VR, Tryder N, Li L, Aja T, Griffith D, Liao FF, Roxas G, Ramprasad MP, Bradshaw C, Barbas CF 3rd (2007) Chemically programmed antibodies: endothelin receptor targeting CovX-Bodies. Bioorg Med Chem Lett 17:501–506
Stanfield RL, Dooley H, Verdino P, Flajnik MF, Wilson IA (2007) Maturation of shark single-domain (IgNAR) antibodies: evidence for induced-fit binding. J Mol Biol 367:358–372
Igawa T, Tsunoda H, Tachibana T, Maeda A, Mimoto F, Moriyama C, Nanami M, Sekimori Y, Nabuchi Y, Aso Y, Hattori K (2010) Reduced elimination of IgG antibodies by engineering the variable region. Protein Eng Des Sel 23:385–392
Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA (2010) Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem 21:2153–2163
Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, Ober RJ, Ward ES (1997) Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol 15:637–640
Gurbaxani B, Dela Cruz LL, Chintalacharuvu K, Morrison SL (2006) Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol Immunol 43:1462–1473
Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, Starovasnik MA, Lowman HB (2009) Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol 182:7663–7671
Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, Lowman HB, Fielder PJ, Prabhu S (2010) Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab Dispos 38:600–605
Wang W, Lu P, Fang P, Hamuro L, Pittman T, Carr B, Hochman J, Prueksaritanont T (2011) Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos 39(9):1469–1477
Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS (2008) Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA 105:9337–9342
Acknowledgments
The author would like to acknowledge the scientific support from the State University of New York at Buffalo under the UB-Pfizer strategic alliance. Author would also like to thank Prof. Joseph P. Balthasar and his past and present lab members for the scientific discussions and impact on the presented work. The authors would also like to thank Hugh Barton and Craig Giragossian for critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Rights and permissions
About this article
Cite this article
Shah, D.K., Betts, A.M. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39, 67–86 (2012). https://doi.org/10.1007/s10928-011-9232-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-011-9232-2