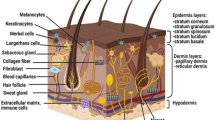
Abstract
The ultimate prospect of tissue engineering is to create autologous tissue grafts for future replacement therapies through utilization of cells and biomaterials simultaneously. Bio-printing is a novel technique, a growing field that is leading to the global revolution in medical sciences that has gained significant attention. Bio-printing has the potential to be used in producing human engineered tissues like bone and skin which then ultimately can be used in the clinics. In this paper, the 3D bio-printing applications of the engineered human tissues that are available (skin and bone) are reviewed. It is evident that various tissue engineering techniques have been applied in the fabrication of skin tissue; therefore, it leads to introduce tissue substitutes such as complementary, split-thickness skin graft, allografts, acellular dermal substitutes and cellularized graft-like commercial products, i.e., Dermagraft and Apligraf. Also, some bone scaffolds based on hydroxyapatite and biphasic calcium phosphate are available in the market. The technology of bio-printing has got validated for bone and skin tissue fabrication, and it is hoped that other tissues could be produced by this technique.



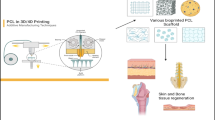
Reproduced with permission from Universidad Carlos III de Madrid (UC3M) and Lawrence [139]. Based on skin wound area, the wound repair strategy is selectable. Whether printing on wound or printing on a feeder layer and assembling it on wound area is available

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Mason C, Dunnill P (2008) A brief definition of regenerative medicine. Regen Med 3(1):1–5. https://doi.org/10.2217/17460751.3.1.1
Abouna GM (2008) Organ shortage crisis: problems and possible solutions. Transpl Proc 40(1):34–38. https://doi.org/10.1016/j.transproceed.2007.11.067
Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R (2014) 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 16:247–276. https://doi.org/10.1146/annurev-bioeng-071813-105155
Mao AS, Mooney DJ (2015) Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci USA 112(47):14452–14459. https://doi.org/10.1073/pnas.1508520112
Orlando G, Booth C, Wang Z, Totonelli G, Ross CL, Moran E, Salvatori M, Maghsoudlou P, Turmaine M, Delario G, Al-Shraideh Y, Farooq U, Farney AC, Rogers J, Iskandar SS, Burns A, Marini FC, De Coppi P, Stratta RJ, Soker S (2013) Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials 34(24):5915–5925. https://doi.org/10.1016/j.biomaterials.2013.04.033
Peloso A, Petrosyan A, Da Sacco S, Booth C, Zambon JP, O’Brien T, Aardema C, Robertson J, De Filippo RE, Soker S, Stratta RJ, Perin L, Orlando G (2015) Renal extracellular matrix scaffolds from discarded kidneys maintain glomerular morphometry and vascular resilience and retains critical growth factors. Transplantation 99(9):1807–1816. https://doi.org/10.1097/tp.0000000000000811
Yu YL, Shao YK, Ding YQ, Lin KZ, Chen B, Zhang HZ, Zhao LN, Wang ZB, Zhang JS, Tang ML, Mei J (2014) Decellularized kidney scaffold-mediated renal regeneration. Biomaterials 35(25):6822–6828. https://doi.org/10.1016/j.biomaterials.2014.04.074
Balestrini JL, Gard AL, Liu A, Leiby KL, Schwan J, Kunkemoeller B, Calle EA, Sivarapatna A, Lin T, Dimitrievska S, Cambpell SG, Niklason LE (2015) Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr Biol 7(12):1598–1610. https://doi.org/10.1039/C5IB00063G
Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC (2014) Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transpl 33(3):298–308. https://doi.org/10.1016/j.healun.2013.10.030
Stabler CT, Lecht S, Mondrinos MJ, Goulart E, Lazarovici P, Lelkes PI (2015) Revascularization of decellularized lung scaffolds: principles and progress. Am J Physiol Lung Cell Mol Physiol 309(11):L1273–L1285. https://doi.org/10.1152/ajplung.00237.2015
Kim TH, Jung Y, Kim SH (2018) Nanofibrous electrospun heart decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng Part A 24(9–10):830–848. https://doi.org/10.1089/ten.TEA.2017.0318
Sánchez PL, Fernández-Santos ME, Costanza S, Climent AM, Moscoso I, Gonzalez-Nicolas MA, Sanz-Ruiz R, Rodríguez H, Kren SM, Garrido G, Escalante JL, Bermejo J, Elizaga J, Menarguez J, Yotti R, Pérez del Villar C, Espinosa MA, Guillem MS, Willerson JT, Bernad A, Matesanz R, Taylor DA, Fernández-Avilés F (2015) Acellular human heart matrix: a critical step toward whole heart grafts. Biomaterials 61:279–289. https://doi.org/10.1016/j.biomaterials.2015.04.056
Seo Y, Jung Y, Kim SH (2018) Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater 67:270–281. https://doi.org/10.1016/j.actbio.2017.11.046
Lee H, Han W, Kim H, Ha DH, Jang J, Kim BS, Cho DW (2017) Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules 18(4):1229–1237. https://doi.org/10.1021/acs.biomac.6b01908
Mattei G, Magliaro C, Pirone A, Ahluwalia A (2017) Decellularized human liver is too heterogeneous for designing a generic extracellular matrix mimic hepatic scaffold. Artif Organs 41(12):E347–E355. https://doi.org/10.1111/aor.12925
Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, Al-Akkad W, Longato L, Brown D, Maghsoudlou P, Dhillon AP, Fuller B, Davidson B, Moore K, Dhar D, De Coppi P, Malago M, Pinzani M (2015) Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep 5:13079. https://doi.org/10.1038/srep13079
Garreta E, Oria R, Tarantino C, Pla-Roca M, Prado P, Fernández-Avilés F, Campistol JM, Samitier J, Montserrat N (2017) Tissue engineering by decellularization and 3D bioprinting. Mater Today 20(4):166–178. https://doi.org/10.1016/j.mattod.2016.12.005
Moser PT, Ott HC (2014) Recellularization of organs: what is the future for solid organ transplantation? Curr Opin Organ Transpl 19(6):603–609. https://doi.org/10.1097/mot.0000000000000131
Cheung DYC, Duan B, Butcher JT (2015) Chapter 21—Bioprinting of cardiac tissues. In: Atala A, Yoo JJ (eds) Essentials of 3D biofabrication and translation. Academic Press, Boston, pp 351–370. https://doi.org/10.1016/B978-0-12-800972-7.00021-9
Groll J, Boland T, Blunk T, Burdick JA, Cho D-W, Dalton PD, Derby B, Forgacs G, Li Q, Mironov VA, Moroni L, Nakamura M, Shu W, Takeuchi S, Vozzi G, Woodfield TBF, Xu T, Yoo JJ, Malda J (2016) Biofabrication: reappraising the definition of an evolving field. Biofabrication 8(1):013001. https://doi.org/10.1088/1758-5090/8/1/013001
Mandrycky C, Wang Z, Kim K, Kim D-H (2016) 3D bioprinting for engineering complex tissues. Biotechnol Adv 34(4):422–434. https://doi.org/10.1016/j.biotechadv.2015.12.011
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785. https://doi.org/10.1038/nbt.2958
Ozbolat IT (2015) Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol 33(7):395–400. https://doi.org/10.1016/j.tibtech.2015.04.005
Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M (2018) 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater 3(2):144–156. https://doi.org/10.1016/j.bioactmat.2017.11.008
Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G (2010) Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2(2):022001. https://doi.org/10.1088/1758-5082/2/2/022001
Moroni L, de Wijn JR, van Blitterswijk CA (2006) 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 27(7):974–985. https://doi.org/10.1016/j.biomaterials.2005.07.023
Zhu W, Ock J, Ma X, Li W, Chen S (2015) “Chapter 2—3D printing and nanomanufacturing. In: Zhang LG, Fisher JP, Leong KW (eds) 3D bioprinting and nanotechnology in tissue engineering and regenerative medicine. Academic Press, New York, pp 25–55. https://doi.org/10.1016/B978-0-12-800547-7.00002-3
Shanjani Y, Pan CC, Elomaa L, Yang Y (2015) A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication 7(4):045008. https://doi.org/10.1088/1758-5090/7/4/045008
Lim G, Choi D, Richardson EB (2014) 3-D printing in organ transplantation. Hanyang Med Rev 34(4):158–164. https://doi.org/10.7599/hmr.2014.34.4.158
Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, Sun W (2011) Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 3(3):034112. https://doi.org/10.1088/1758-5082/3/3/034112
Perkins JD (2007) Are we reporting the same thing? Liver Transpl: Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc 13(3):465–466
Midha S, Dalela M, Sybil D, Patra P, Mohanty S (2019) Advances in three-dimensional bioprinting of bone: progress and challenges. J Tissue Eng Regen Med 13(6):925–945. https://doi.org/10.1002/term.2847
Moreno Madrid AP, Vrech SM, Sanchez MA, Rodriguez AP (2019) Advances in additive manufacturing for bone tissue engineering scaffolds. Mater Sci Eng, C 100:631–644. https://doi.org/10.1016/j.msec.2019.03.037
Ashammakhi N, Hasan A, Kaarela O, Byambaa B, Sheikhi A, Gaharwar AK, Khademhosseini A (2019) Advancing frontiers in bone bioprinting. Adv Healthc Mater 8(7):1801048. https://doi.org/10.1002/adhm.201801048
Atala A, Forgacs G (2019) Three-dimensional bioprinting in regenerative medicine: reality, hype, and future. Stem Cells Transl Med 8(8):744–745. https://doi.org/10.1002/sctm.19-0089
Dasgupta Q, Black LD (2019) A fresh slate for 3D bioprinting. Science 365(6452):446. https://doi.org/10.1126/science.aay0478
Kuss M, Duan B (2019) Chapter 2—Extrusion-based bioprinting. In: Cho D-W (ed) Biofabrication and 3D tissue modeling. The Royal Society of Chemistry, London, pp 22–48. https://doi.org/10.1039/9781788012683-00022
Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT (2019) Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226:119536. https://doi.org/10.1016/j.biomaterials.2019.119536
Miri AK, Khalilpour A, Cecen B, Maharjan S, Shin SR, Khademhosseini A (2019) Multiscale bioprinting of vascularized models. Biomaterials 198:204–216. https://doi.org/10.1016/j.biomaterials.2018.08.006
Moldovan F (2019) Recent trends in bioprinting. Procedia Manuf 32:95–101. https://doi.org/10.1016/j.promfg.2019.02.188
Shafiee A, Ghadiri E, Ramesh H, Kengla C, Kassis J, Calvert P, Williams D, Khademhosseini A, Narayan R, Forgacs G, Atala A (2019) Physics of bioprinting. Appl Phys Rev 6(2):021315. https://doi.org/10.1063/1.5087206
Zhang B, Gao L, Ma L, Luo Y, Yang H, Cui Z (2019) 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering 5(4):777–794. https://doi.org/10.1016/j.eng.2019.03.009
Zhou D, Chen J, Liu B, Zhang X, Li X, Xu T (2019) Bioinks for jet-based bioprinting. Bioprinting 16:e00060. https://doi.org/10.1016/j.bprint.2019.e00060
Unagolla JM, Jayasuriya AC (2019) Hydrogel-based 3D bioprinting: a comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl Mater Today. https://doi.org/10.1016/j.apmt.2019.100479
Liu P, Shen H, Zhi Y, Si J, Shi J, Guo L, Shen SG (2019) 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf, B 181:1026–1034. https://doi.org/10.1016/j.colsurfb.2019.06.069
Cheng L, Yao B, Hu T, Cui X, Shu X, Tang S, Wang R, Wang Y, Liu Y, Song W, Fu X, Li H, Huang S (2019) Properties of an alginate-gelatin-based bioink and its potential impact on cell migration, proliferation, and differentiation. Int J Biol Macromol 135:1107–1113. https://doi.org/10.1016/j.ijbiomac.2019.06.017
Gonzalez-Fernandez T, Rathan S, Hobbs C, Pitacco P, Freeman FE, Cunniffe GM, Dunne NJ, McCarthy HO, Nicolosi V, O’Brien FJ, Kelly DJ (2019) Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J Control Release 301:13–27. https://doi.org/10.1016/j.jconrel.2019.03.006
Kim W, Kim G (2019) A functional bioink and its application in myoblast alignment and differentiation. Chem Eng J 366:150–162. https://doi.org/10.1016/j.cej.2019.02.071
Oliveira EP, Malysz-Cymborska I, Golubczyk D, Kalkowski L, Kwiatkowska J, Reis RL, Oliveira JM, Walczak P (2019) Advances in bioinks and in vivo imaging of biomaterials for CNS applications. Acta Biomater 95:60–72. https://doi.org/10.1016/j.actbio.2019.05.006
Kajave NS, Schmitt T, Nguyen T-U, Kishore V (2019) Dual crosslinking strategy to generate mechanically viable cell-laden printable constructs using methacrylated collagen bioinks. Materials Science and Engineering: C 107:110290. https://doi.org/10.1016/j.msec.2019.110290
Zhu K, Shin SR, van Kempen T, Li Y-C, Ponraj V, Nasajpour A, Mandla S, Hu N, Liu X, Leijten J, Lin Y-D, Hussain MA, Zhang YS, Tamayol A, Khademhosseini A (2017) Gold nanocomposite bioink for printing 3D cardiac constructs. Adv Func Mater 27(12):1605352. https://doi.org/10.1002/adfm.201605352
Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F, Enquist LW, Jia X, McAlpine MC (2015) 3D printed anatomical nerve regeneration pathways. Adv Funct Mater 25(39):6205–6217. https://doi.org/10.1002/adfm.201501760
Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA (2016) Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6:34845. https://doi.org/10.1038/srep34845. https://www.nature.com/articles/srep34845#supplementary-information
Kesti M, Eberhardt C, Pagliccia G, Kenkel D, Grande D, Boss A, Zenobi-Wong M (2015) Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv Funct Mater 25(48):7406–7417. https://doi.org/10.1002/adfm.201503423
Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, Verma N, Gracias DH, McAlpine MC (2013) 3D printed bionic ears. Nano Lett 13(6):2634–2639. https://doi.org/10.1021/nl4007744
Khan Y, Pavinatto FJ, Lin MC, Liao A, Swisher SL, Mann K, Subramanian V, Maharbiz MM, Arias AC (2016) Inkjet-printed flexible gold electrode arrays for bioelectronic interfaces. Adv Funct Mater 26(7):1004–1013. https://doi.org/10.1002/adfm.201503316
Shin SR, Farzad R, Tamayol A, Manoharan V, Mostafalu P, Zhang YS, Akbari M, Jung SM, Kim D, Comotto M, Annabi N, Al-Hazmi FE, Dokmeci MR, Khademhosseini A (2016) A bioactive carbon nanotube-based ink for printing 2D and 3D flexible electronics. Adv Mater 28(17):3280–3289. https://doi.org/10.1002/adma.201506420
Coruh A, Yontar Y (2012) Application of split-thickness dermal grafts in deep partial- and full-thickness burns: a new source of auto-skin grafting. J Burn Care Res: Off Publ Am Burn Assoc 33(3):e94–e100. https://doi.org/10.1097/BCR.0b013e31823499e9
Leon-Villapalos J, Eldardiri M, Dziewulski P (2010) The use of human deceased donor skin allograft in burn care. Cell Tissue Bank 11(1):99–104. https://doi.org/10.1007/s10561-009-9152-1
Metcalfe AD, Ferguson MWJ (2007) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4(14):413–437. https://doi.org/10.1098/rsif.2006.0179
Pourchet LJ, Thepot A, Albouy M, Courtial EJ, Boher A, Blum LJ, Marquette CA (2017) Human skin 3D bioprinting using scaffold-free approach. Adv Healthc Mater. https://doi.org/10.1002/adhm.201601101
Cubo N, Garcia M, del Cañizo JF, Velasco D, Jorcano JL (2016) 3D bioprinting of functional human skin: production andin vivoanalysis. Biofabrication 9(1):015006. https://doi.org/10.1088/1758-5090/9/1/015006
Zhao X, Lang Q, Yildirimer L, Lin ZY, Cui W, Annabi N, Ng KW, Dokmeci MR, Ghaemmaghami AM, Khademhosseini A (2016) photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv Healthc Mater 5(1):108–118. https://doi.org/10.1002/adhm.201500005
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo S-S, Dai G, Karande P (2014) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C, Methods 20(6):473–484. https://doi.org/10.1089/ten.TEC.2013.0335
Xiong S, Zhang X, Lu P, Wu Y, Wang Q, Sun H, Heng BC, Bunpetch V, Zhang S, Ouyang H (2017) A gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci Rep 7(1):4288. https://doi.org/10.1038/s41598-017-04149-y
Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN (2015) A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater 27(9):1607–1614. https://doi.org/10.1002/adma.201405076
Ng WL, Yeong WY, Naing MW (2016) Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int J Biopr 2(1):10. https://doi.org/10.18063/ijb.2016.01.009
Min D, Lee W, Bae IH, Lee TR, Croce P, Yoo SS (2018) Bioprinting of biomimetic skin containing melanocytes. Exp Dermatol 27(5):453–459. https://doi.org/10.1111/exd.13376
Li J, Chi J, Liu J, Gao C, Wang K, Shan T, Li Y, Shang W, Gu F (2017) 3D printed gelatin-alginate bioactive scaffolds combined with mice bone marrow mesenchymal stem cells: a biocompatibility study. Int J Clin Exp Pathol 10(6):6299–6307
Huang S, Yao B, Xie J, Fu X (2016) 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater 32:170–177. https://doi.org/10.1016/j.actbio.2015.12.039
Ng WL, Yeong WY, Win Naing M (2014) Potential of bioprinted films for skin tissue engineering. In: Paper presented at the 1st international conference on progress in additive manufacturing
Rimann M, Bono E, Annaheim H, Bleisch M, Graf-Hausner U (2016) Standardized 3D bioprinting of soft tissue models with human primary cells. J Lab Autom 21(4):496–509. https://doi.org/10.1177/2211068214567146
Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S (2012) Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem cells Transl Med 1(11):792–802. https://doi.org/10.5966/sctm.2012-0088
Kim BS, Lee J-S, Gao G, Cho D-W (2017) Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 9(2):025034. https://doi.org/10.1088/1758-5090/aa71c8
Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K (2013) Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 8(3):e57741
Binder KW (2011) In situ bioprinting of the skin. Wake Forest University, Winston-Salem
Thayer PS, Orrhult LS, Martínez H (2018) Bioprinting of cartilage and skin tissue analogs utilizing a novel passive mixing unit technique for bioink precellularization. J Vis Exp JoVE 3(131):56372. https://doi.org/10.3791/56372
Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, Zhao W, Tan J, El-Amin IB, Dice DD, Marco J, Green J, Xu T, Skardal A, Holmes JH, Jackson JD, Atala A, Yoo JJ (2019) In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep 9(1):1856. https://doi.org/10.1038/s41598-018-38366-w
Admane P, Gupta AC, Jois P, Roy S, Chandrasekharan Lakshmanan C, Kalsi G, Bandyopadhyay B, Ghosh S (2019) Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting 15:e00051. https://doi.org/10.1016/j.bprint.2019.e00051
Mao JS, Zhao LG, Yin YJ, Yao KD (2003) Structure and properties of bilayer chitosan-gelatin scaffolds. Biomaterials 24(6):1067–1074
Leukers B, Gülkan H, Irsen SH, Milz S, Tille C, Schieker M, Seitz H (2005) Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med 16(12):1121–1124. https://doi.org/10.1007/s10856-005-4716-5
Cox SC, Thornby JA, Gibbons GJ, Williams MA, Mallick KK (2015) 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater Sci Eng, C 47:237–247. https://doi.org/10.1016/j.msec.2014.11.024
Brunello G, Sivolella S, Meneghello R, Ferroni L, Gardin C, Piattelli A, Zavan B, Bressan E (2016) Powder-based 3D printing for bone tissue engineering. Biotechnol Adv 34(5):740–753. https://doi.org/10.1016/j.biotechadv.2016.03.009
Murphy C, Kolan K, Li W, Semon J, Day D, Leu M (2017) 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int J Biopr 3(1):11. https://doi.org/10.18063/ijb.2017.01.005
Byambaa B, Annabi N, Yue K, Trujillo-de Santiago G, Alvarez MM, Jia W, Kazemzadeh-Narbat M, Shin SR, Tamayol A, Khademhosseini A (2017) Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. https://doi.org/10.1002/adhm.201700015
Kim MS, Kim G (2014) Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr Polym 114:213–221. https://doi.org/10.1016/j.carbpol.2014.08.008
Holmes B, Bulusu K, Plesniak M, Zhang LG (2016) A synergistic approach to the design, fabrication and evaluation of 3D printed micro and nano featured scaffolds for vascularized bone tissue repair. Nanotechnology 27(6):064001. https://doi.org/10.1088/0957-4484/27/6/064001
Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X (2014) Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J 9(10):1304–1311. https://doi.org/10.1002/biot.201400305
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319. https://doi.org/10.1038/nbt.3413
Wang J, Yang M, Zhu Y, Wang L, Tomsia AP, Mao C (2014) Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv Mater 26(29):4961–4966. https://doi.org/10.1002/adma.201400154
Costantini M, Idaszek J, Szoke K, Jaroszewicz J, Dentini M, Barbetta A, Brinchmann JE, Swieszkowski W (2016) 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 8(3):035002. https://doi.org/10.1088/1758-5090/8/3/035002
Kim HH, Park JB, Kang MJ, Park YH (2014) Surface-modified silk hydrogel containing hydroxyapatite nanoparticle with hyaluronic acid-dopamine conjugate. Int J Biol Macromol 70:516–522. https://doi.org/10.1016/j.ijbiomac.2014.06.052
Bendtsen ST, Quinnell SP, Wei M (2017) Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J Biomed Mater Res A 105(5):1457–1468. https://doi.org/10.1002/jbm.a.36036
Nyberg E, Rindone A, Dorafshar A, Grayson WL (2017) Comparison of 3D-printed poly-varepsilon-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Eng Part A 23(11–12):503–514. https://doi.org/10.1089/ten.TEA.2016.0418
Buyuksungur S, Endogan Tanir T, Buyuksungur A, Bektas EI, Torun Kose G, Yucel D, Beyzadeoglu T, Cetinkaya E, Yenigun C, Tonuk E, Hasirci V, Hasirci N (2017) 3D printed poly(epsilon-caprolactone) scaffolds modified with hydroxyapatite and poly(propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater Sci 5(10):2144–2158. https://doi.org/10.1039/c7bm00514h
Bose S, Vahabzadeh S, Bandyopadhyay A (2013) Bone tissue engineering using 3D printing. Mater Today 16(12):496–504. https://doi.org/10.1016/j.mattod.2013.11.017
Daly AC, Pitacco P, Nulty J, Cunniffe GM, Kelly DJ (2018) 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 162:34–46. https://doi.org/10.1016/j.biomaterials.2018.01.057
Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ (2012) Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C, Methods 18(1):33–44. https://doi.org/10.1089/ten.TEC.2011.0060
Huang B, Bártolo PJ (2018) Rheological characterization of polymer/ceramic blends for 3D printing of bone scaffolds. Polym Test 68:365–378. https://doi.org/10.1016/j.polymertesting.2018.04.033
Hung BP, Naved BA, Nyberg EL, Dias M, Holmes CA, Elisseeff JH, Dorafshar AH, Grayson WL (2016) Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater Sci Eng 2(10):1806–1816. https://doi.org/10.1021/acsbiomaterials.6b00101
Kebede MA, Asiku KS, Imae T, Kawakami M, Furukawa H, Wu CM (2018) Stereolithographic and molding fabrications of hydroxyapatite-polymer gels applicable to bone regeneration materials. J Taiwan Inst Chem Eng 92:91–96. https://doi.org/10.1016/j.jtice.2018.01.034
Khanarian NT, Jiang J, Wan LQ, Mow VC, Lu HH (2012) A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A 18(5–6):533–545. https://doi.org/10.1089/ten.TEA.2011.0279
Kim YC, Min KH, Choi JW, Koh KS, Oh TS, Jeong WS (2018) Patient-specific puzzle implant preformed with 3D-printed rapid prototype model for combined orbital floor and medial wall fracture. J Plast Reconstr Aesthet Surg: JPRAS 71(4):496–503. https://doi.org/10.1016/j.bjps.2017.11.016
Lee H, Yang GH, Kim M, Lee J, Huh J, Kim G (2018) Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater Sci Eng, C 84:140–147. https://doi.org/10.1016/j.msec.2017.11.013
Luo Y, Lode A, Wu C, Chang J, Gelinsky M (2015) Alginate/nanohydroxyapatite scaffolds with designed core/shell structures fabricated by 3D plotting and in situ mineralization for bone tissue engineering. ACS Appl Mater Interfaces 7(12):6541–6549. https://doi.org/10.1021/am508469h
Müller M, Becher J, Schnabelrauch M, Zenobi-Wong M (2013) Printing thermoresponsive reverse molds for the creation of patterned two-component hydrogels for 3D cell culture. JoVE 77:e50632. https://doi.org/10.3791/50632
Ni J, Li D, Mao M, Dang X, Wang K, He J, Shi Z (2018) A method of accurate bone tunnel placement for anterior cruciate ligament reconstruction based on 3-dimensional printing technology: a cadaveric study. Arthroscopy 34(2):546–556. https://doi.org/10.1016/j.arthro.2017.08.288
Park J, Lee SJ, Lee H, Park SA, Lee JY (2018) Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydr Polym 196:217–224. https://doi.org/10.1016/j.carbpol.2018.05.048
Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH (2008) In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res, Part A 86(1):1–12. https://doi.org/10.1002/jbm.a.32073
Tayebi L, Rasoulianboroujeni M, Moharamzadeh K, Almela TKD, Cui Z, Ye H (2018) 3D-printed membrane for guided tissue regeneration. Mater Sci Eng, C 84:148–158. https://doi.org/10.1016/j.msec.2017.11.027
Trauner KB (2018) The emerging role of 3D printing in arthroplasty and orthopedics. J Arthroplasty 33(8):2352–2354. https://doi.org/10.1016/j.arth.2018.02.033
Zhang B, Pei X, Zhou C, Fan Y, Jiang Q, Ronca A, D’Amora U, Chen Y, Li H, Sun Y, Zhang X (2018) The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4 V scaffold for load-bearing bone reconstruction. Mater Des 152:30–39. https://doi.org/10.1016/j.matdes.2018.04.065
Hernández-González AC, Téllez-Jurado L, Rodríguez-Lorenzo LM (2019) Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: a review. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115514
Oladapo BI, Zahedi SA, Adeoye AOM (2019) 3D printing of bone scaffolds with hybrid biomaterials. Compos B Eng 158:428–436. https://doi.org/10.1016/j.compositesb.2018.09.065
Zhao L, Pei X, Jiang L, Hu C, Sun J, Xing F, Zhou C, Fan Y, Zhang X (2019) Bionic design and 3D printing of porous titanium alloy scaffolds for bone tissue repair. Compos B Eng 162:154–161. https://doi.org/10.1016/j.compositesb.2018.10.094
Lai Y, Li Y, Cao H, Long J, Wang X, Li L, Li C, Jia Q, Teng B, Tang T, Peng J, Eglin D, Alini M, Grijpma DW, Richards G, Qin L (2019) Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 197:207–219. https://doi.org/10.1016/j.biomaterials.2019.01.013
Roopavath UK, Malferrari S, Van Haver A, Verstreken F, Rath SN, Kalaskar DM (2019) Optimization of extrusion based ceramic 3D printing process for complex bony designs. Mater Des 162:263–270. https://doi.org/10.1016/j.matdes.2018.11.054
Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ (2002) Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 99(16):10730–10735. https://doi.org/10.1073/pnas.162349799
Kaully T, Kaufman-Francis K, Lesman A, Levenberg S (2009) Vascularization–the conduit to viable engineered tissues. Tissue Eng Part B, Rev 15(2):159–169. https://doi.org/10.1089/ten.teb.2008.0193
Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC (2001) Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res 55(2):203–216
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18(11–12):1304–1312. https://doi.org/10.1089/ten.TEA.2011.0543
Dolati F, Yu Y, Zhang Y, De Jesus AM, Sander EA, Ozbolat IT (2014) In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology 25(14):145101. https://doi.org/10.1088/0957-4484/25/14/145101
Duan B, Hockaday LA, Kang KH, Butcher JT (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res, Part A 101(5):1255–1264. https://doi.org/10.1002/jbm.a.34420
Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amedee J, Fricain JC, Catros S (2010) In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication 2(1):014101. https://doi.org/10.1088/1758-5082/2/1/014101
Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, Atala A (2013) Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5(1):015001. https://doi.org/10.1088/1758-5082/5/1/015001
Zhang T, Yan KC, Ouyang L, Sun W (2013) Mechanical characterization of bioprinted in vitro soft tissue models. Biofabrication 5(4):045010. https://doi.org/10.1088/1758-5082/5/4/045010
Duarte Campos DF, Blaeser A, Weber M, Jakel J, Neuss S, Jahnen-Dechent W, Fischer H (2013) Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 5(1):015003. https://doi.org/10.1088/1758-5082/5/1/015003
Gruene M, Pflaum M, Deiwick A, Koch L, Schlie S, Unger C, Wilhelmi M, Haverich A, Chichkov BN (2011) Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication 3(1):015005. https://doi.org/10.1088/1758-5082/3/1/015005
Hong S, Song SJ, Lee JY, Jang H, Choi J, Sun K, Park Y (2013) Cellular behavior in micropatterned hydrogels by bioprinting system depended on the cell types and cellular interaction. J Biosci Bioeng 116(2):224–230. https://doi.org/10.1016/j.jbiosc.2013.02.011
Owens CM, Marga F, Forgacs G, Heesch CM (2013) Biofabrication and testing of a fully cellular nerve graft. Biofabrication 5(4):045007. https://doi.org/10.1088/1758-5082/5/4/045007
Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJ, Melchels FP, Malda J (2013) Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 5(3):035007. https://doi.org/10.1088/1758-5082/5/3/035007
Xu F, Sridharan B, Wang S, Gurkan UA, Syverud B, Demirci U (2011) Embryonic stem cell bioprinting for uniform and controlled size embryoid body formation. Biomicrofluidics 5(2):22207. https://doi.org/10.1063/1.3580752
Kannan S (2014) The 3D bio printing revolution. Harv Sci Rev. https://harvardsciencereview.com/2014/05/01/the-3d-bioprinting-revolution/
Arslan-Yildiz A, El Assal R, Chen P, Guven S, Inci F, Demirci U (2016) Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8(1):014103. https://doi.org/10.1088/1758-5090/8/1/014103
Vaidya M (2015) Startups tout commercially 3D-printed tissue for drug screening. Nat Med 21(1):2. https://doi.org/10.1038/nm0115-2
Wang CC, Yang KC, Lin KH, Liu HC, Lin FH (2011) A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 32(29):7118–7126. https://doi.org/10.1016/j.biomaterials.2011.06.018
Chang R, Emami K, Wu H, Sun W (2010) Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2(4):045004. https://doi.org/10.1088/1758-5082/2/4/045004
Singh S, Choudhury D, Yu F, Mironov V, Naing MW (2019) In situ bioprinting–bioprinting from benchside to bedside? Acta Biomater. https://doi.org/10.1016/j.actbio.2019.08.045
Murr LE (2015) Bioprinting and biofabrication of organs. In: Handbook of materials structures, properties, processing and performance. Springer, Cham, pp 629–638. https://doi.org/10.1007/978-3-319-01815-7_36
Acknowledgements
A word of appreciation must be given to Aron Munggela Foma for his invaluable assistance and comments on the first phase of paper editing. Also, we are extremely grateful to Mariam Sharifi Sistani for her precision and insightful comments on the second draft edition.
Funding
This study was not funded by any institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beheshtizadeh, N., Lotfibakhshaiesh, N., Pazhouhnia, Z. et al. A review of 3D bio-printing for bone and skin tissue engineering: a commercial approach. J Mater Sci 55, 3729–3749 (2020). https://doi.org/10.1007/s10853-019-04259-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04259-0