Abstract
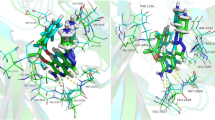
Anaplastic lymphoma kinase (ALK) has been thought to be a prospective target of anti-drug resistance design in treatment of tumors and specific neuron diseases. It is highly useful for the seeking of possible strategy alleviating drug resistance to probe the mutation-mediated effect on binding of inhibitors to ALK. In the current work, multiple replica Gaussian accelerated molecular dynamics (MR-GaMD) simulations, molecular mechanics generalized Born surface area (MM-GBSA) and free energy landscapes were coupled to explore influences of mutations L1198F, L1198F/C1156Y, and C1156Y on the binding of the first ALK inhibitor crizotinib to ALK. The results suggest that three mutations obviously affect structural flexibility, motion modes and conformational changes of ALKs. L1198F and L1198F/C1156Y strengthen the binding of crizotinib to the mutated ALKs but C1156Y induces evident drug resistance toward crizotinib. Analyses of free energy landscapes show that stability in the orientation and positions of crizotinib relative to ALK plays a vital role in alleviating drug resistance of mutations toward crizotinib. Residue-based free energy decomposition method was utilized to evaluate the contributions of separate residues to the binding of crizotinib. The results not only indicate that the tuning of point mutation L1198F on interaction networks of crizotinib with ALK can be regarded as a possible strategy to relieve drug resistance of the mutated ALK but also further verify that residues L1122, V1130, L1196, L1198, M1199, and L1256 can be used as efficient targets of anti-drug resistance design induced by mutations.










Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP (1997) ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 14(18):2175–2188
Bilsland JG, Wheeldon A, Mead A, Znamenskiy P, Almond S, Waters KA, Thakur M, Beaumont V, Bonnert TP, Heavens R, Whiting P, McAllister G, Munoz-Sanjuan I (2008) Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology 33(3):685–700
Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G (2008) The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 8(1):11–23
Webb TR, Slavish J, George RE, Look AT, Xue L, Jiang Q, Cui X, Rentrop WB, Morris SW (2009) Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther 9(3):331–356
Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T (1997) Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14(4):439–449
Morris S, Kirstein M, Valentine M, Dittmer K, Shapiro D, Saltman D, Look A (1994) Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 263(5151):1281–1284
Yu H, Huang J-x, Wang C-f, Shi D-r (2011) ALK-positive large B-cell lymphoma: report of a case. Zhonghua bing li xue za zhi 40(8):561–562
Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ (1999) Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res 59(12):2776–2780
Mano H (2008) Non-solid oncogenes in solid tumors: EML4–ALK fusion genes in lung cancer. Cancer Sci 99(12):2349–2355
Shaw AT, Solomon B (2011) Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res 17(8):2081–2086
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S-i, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H (2007) Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 448(7153):561–566
Roskoski R (2013) Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol Res 68(1):68–94
Johnson TW, Richardson PF, Bailey S, Brooun A, Burke BJ, Collins MR, Cui JJ, Deal JG, Deng Y-L, Dinh D, Engstrom LD, He M, Hoffman J, Hoffman RL, Huang Q, Kania RS, Kath JC, Lam H, Lam JL, Le PT, Lingardo L, Liu W, McTigue M, Palmer CL, Sach NW, Smeal T, Smith GL, Stewart AE, Timofeevski S, Zhu H, Zhu J, Zou HY, Edwards MP (2014) Discovery of (10R)-7-Amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a Macrocyclic Inhibitor of Anaplastic Lymphoma Kinase (ALK) and c-ros Oncogene 1 (ROS1) with Preclinical Brain Exposure and Broad-Spectrum Potency against ALK-Resistant Mutations. J Med Chem 57(11):4720–4744
Michellys P-Y, Chen B, Jiang T, Jin Y, Lu W, Marsilje TH, Pei W, Uno T, Zhu X, Wu B, Nguyen TN, Bursulaya B, Lee C, Li N, Kim S, Tuntland T, Liu B, Sun F, Steffy A, Hood T (2016) Design and synthesis of novel selective anaplastic lymphoma kinase inhibitors. Bioorg Med Chem Lett 26(3):1090–1096
Epstein LF, Chen H, Emkey R, Whittington DA (2012) The R1275Q neuroblastoma mutant and certain ATP-competitive inhibitors stabilize alternative activation loop conformations of anaplastic lymphoma kinase. J Biol Chem 287(44):37447–37457
Tu C-H, Lin W-H, Peng Y-H, Hsu T, Wu J-S, Chang C-Y, Lu C-T, Lyu P-C, Shih C, Jiaang W-T, Wu S-Y (2016) Pyrazolylamine derivatives reveal the conformational switching between type I and type ii binding modes of anaplastic lymphoma kinase (ALK). J Med Chem 59(8):3906–3919
Huang W-S, Liu S, Zou D, Thomas M, Wang Y, Zhou T, Romero J, Kohlmann A, Li F, Qi J, Cai L, Dwight TA, Xu Y, Xu R, Dodd R, Toms A, Parillon L, Lu X, Anjum R, Zhang S, Wang F, Keats J, Wardwell SD, Ning Y, Xu Q, Moran LE, Mohemmad QK, Jang HG, Clackson T, Narasimhan NI, Rivera VM, Zhu X, Dalgarno D, Shakespeare WC (2016) Discovery of Brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem 59(10):4948–4964
Zhang P, Dong J, Zhong B, Zhang D, Yuan H, Jin C, Xu X, Li H, Zhou Y, Liang Z, Ji M, Xu T, Song G, Zhang L, Chen G, Meng X, Sun D, Shih J, Zhang R, Hou G, Wang C, Jin Y, Yang Q (2016) Design and synthesis of novel 3-sulfonylpyrazol-4-amino pyrimidines as potent anaplastic lymphoma kinase (ALK) inhibitors. Bioorg Med Chem Lett 26(8):1910–1918
Lee Christian C, Jia Y, Li N, Sun X, Ng K, Ambing E, Gao M-Y, Hua S, Chen C, Kim S, Michellys P-Y, Lesley Scott A, Harris Jennifer L, Spraggon G (2010) Crystal structure of the ALK (anaplastic lymphoma kinase) catalytic domain. Biochem J 430(3):425–437
Bryan MC, Whittington DA, Doherty EM, Falsey JR, Cheng AC, Emkey R, Brake RL, Lewis RT (2012) Rapid development of piperidine carboxamides as potent and selective anaplastic lymphoma kinase inhibitors. J Med Chem 55(4):1698–1705
Johnson TW, Bolanos B, Brooun A, Gallego RA, Gehlhaar D, Jalaie M, McTigue M, Timofeevski S (2018) Reviving B-factors: activating ALK mutations increase protein dynamics of the unphosphorylated kinase. ACS Med Chem Lett 9(9):872–877
Gummadi VR, Rajagopalan S, Looi C-Y, Paydar M, Renukappa GA, Ainan BR, Krishnamurthy NR, Panigrahi SK, Mahasweta K, Raghuramachandran S, Rajappa M, Ramanathan A, Lakshminarasimhan A, Ramachandra M, Wong P-F, Mustafa MR, Nanduri S, Hosahalli S (2013) Discovery of 7-azaindole based anaplastic lymphoma kinase (ALK) inhibitors: Wild type and mutant (L1196M) active compounds with unique binding mode. Bioorg Med Chem Lett 23(17):4911–4918
Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung P-P, Pairish M, Jia L, Meng J, Funk L, Botrous I, McTigue M, Grodsky N, Ryan K, Padrique E, Alton G, Timofeevski S, Yamazaki S, Li Q, Zou H, Christensen J, Mroczkowski B, Bender S, Kania RS, Edwards MP (2011) Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 54(18):6342–6363
Marsilje TH, Pei W, Chen B, Lu W, Uno T, Jin Y, Jiang T, Kim S, Li N, Warmuth M, Sarkisova Y, Sun F, Steffy A, Pferdekamper AC, Li AG, Joseph SB, Kim Y, Liu B, Tuntland T, Cui X, Gray NS, Steensma R, Wan Y, Jiang J, Chopiuk G, Li J, Gordon WP, Richmond W, Johnson K, Chang J, Groessl T, He Y-Q, Phimister A, Aycinena A, Lee CC, Bursulaya B, Karanewsky DS, Seidel HM, Harris JL, Michellys P-Y (2013) Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in Phase 1 and Phase 2 Clinical Trials. J Med Chem 56(14):5675–5690
Kim D-W, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, Huber RM, West HL, Groen HJM, Hochmair MJ, Leighl NB, Gettinger SN, Langer CJ, Rodríguez LGPA, Smit EF, Kim ES, Reichmann W, Haluska FG, Kerstein D, Camidge DR (2017) Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol.
Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, Fujita N, Katayama R (2017) Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 8(1):14768
Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, Chao BH, Borghaei H, Gold KA, Zeaiter A, Bordogna W, Balas B, Puig O, Henschel V, Ou S-HI (2016) Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 17(2):234–242
Mori M, Ueno Y, Konagai S, Fushiki H, Shimada I, Kondoh Y, Saito R, Mori K, Shindou N, Soga T, Sakagami H, Furutani T, Doihara H, Kudoh M, Kuromitsu S (2014) The selective anaplastic lymphoma receptor tyrosine kinase inhibitor ASP3026 induces tumor regression and prolongs survival in non-small cell lung cancer model mice. Mol Cancer Ther 13(2):329–340
Cheung N-KV, Dyer MA (2013) Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 13(6):397–411
Huang Q, Johnson TW, Bailey S, Brooun A, Bunker KD, Burke BJ, Collins MR, Cook AS, Cui JJ, Dack KN, Deal JG, Deng Y-L, Dinh D, Engstrom LD, He M, Hoffman J, Hoffman RL, Johnson PS, Kania RS, Lam H, Lam JL, Le PT, Li Q, Lingardo L, Liu W, Lu MW, McTigue M, Palmer CL, Richardson PF, Sach NW, Shen H, Smeal T, Smith GL, Stewart AE, Timofeevski S, Tsaparikos K, Wang H, Zhu H, Zhu J, Zou HY, Edwards MP (2014) Design of potent and selective inhibitors to overcome clinical anaplastic lymphoma kinase mutations resistant to crizotinib. J Med Chem 57(4):1170–1187
Nagasundaram N, Wilson Alphonse CR, Samuel Gnana PV, Rajaretinam RK (2017) Molecular dynamics validation of crizotinib resistance to ALK mutations (L1196M and G1269A) and identification of specific inhibitors. J Cell Biochem 118(10):3462–3471
Shaw AT, Friboulet L, Leshchiner I, Gainor JF, Bergqvist S, Brooun A, Burke BJ, Deng Y-L, Liu W, Dardaei L, Frias RL, Schultz KR, Logan J, James LP, Smeal T, Timofeevski S, Katayama R, Iafrate AJ, Le L, McTigue M, Getz G, Johnson TW, Engelman JA (2015) Resensitization to crizotinib by the Lorlatinib ALK resistance mutation L1198F. New Engl J Med 374(1):54–61
Janoueix-Lerosey I, Lequin D, Brugières L, Ribeiro A, de Pontual L, Combaret V, Raynal V, Puisieux A, Schleiermacher G, Pierron G, Valteau-Couanet D, Frebourg T, Michon J, Lyonnet S, Amiel J, Delattre O (2008) Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455(7215):967–970
Bresler Scott C, Weiser Daniel A, Huwe Peter J, Park Jin H, Krytska K, Ryles H, Laudenslager M, Rappaport Eric F, Wood Andrew C, McGrady Patrick W, Hogarty Michael D, London Wendy B, Radhakrishnan R, Lemmon Mark A, Mossé Yaël P (2014) ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell 26(5):682–694
Mossé YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM (2008) Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455(7215):930–935
Fushimi M, Fujimori I, Wakabayashi T, Hasui T, Kawakita Y, Imamura K, Kato T, Murakami M, Ishii T, Kikko Y, Kasahara M, Nakatani A, Hiura Y, Miyamoto M, Saikatendu K, Zou H, Lane SW, Lawson JD, Imoto H (2019) Discovery of potent, selective, and brain-penetrant 1H-Pyrazol-5-yl-1H-pyrrolo[2,3-b]pyridines as anaplastic lymphoma kinase (ALK) inhibitors. J Med Chem 62(10):4915–4935
Xue W, Yang F, Wang P, Zheng G, Chen Y, Yao X, Zhu F (2018) What contributes to serotonin-norepinephrine reuptake inhibitors’ dual-targeting mechanism? The key role of transmembrane domain 6 in human serotonin and norepinephrine transporters revealed by molecular dynamics simulation. ACS Chem Neurosci 9(5):1128–1140
Fu T, Zheng G, Tu G, Yang F, Chen Y, Yao X, Li X, Xue W, Zhu F (2018) Exploring the binding mechanism of metabotropic glutamate receptor 5 negative allosteric modulators in clinical trials by molecular dynamics simulations. ACS Chem Neurosci 9(6):1492–1502
Yang M-J, Pang X-Q, Zhang X, Han K-L (2011) Molecular dynamics simulation reveals preorganization of the chloroplast FtsY towards complex formation induced by GTP binding. J Struct Biol 173(1):57–66
Xu Y, Li S, Yan Z, Ge B, Huang F, Yue T (2019) Revealing cooperation between knotted conformation and dimerization in protein stabilization by molecular dynamics simulations. J Phys Chem Lett 10(19):5815–5822
Zhang S, Lin X (2019) Lipid acyl chain cis double bond position modulates membrane domain registration/anti-registration. J Am Chem Soc 141(40):15884–15890
Hu G, Yu X, Bian Y, Cao Z, Xu S, Zhao L, Ji B, Wang W, Wang J (2018) Atomistic analysis of ToxN and ToxI complex unbinding mechanism. Int J Mol Sci 19(11):3524
Wang R-G, Zhang H-X, Zheng Q-C (2020) Revealing the binding and drug resistance mechanism of amprenavir, indinavir, ritonavir, and nelfinavir complexed with HIV-1 protease due to double mutations G48T/L89M by molecular dynamics simulations and free energy analyses. Phys Chem Chem Phys 22(8):4464–4480
Wu EL, Han K, Zhang JZH (2008) Selectivity of neutral/weakly basic P1 group inhibitors of thrombin and trypsin by a molecular dynamics study. Chem Eur J 14(28): 8704–8714
Chen J, Wang X, Pang L, Zhang JZH, Zhu T (2019) Effect of mutations on binding of ligands to guanine riboswitch probed by free energy perturbation and molecular dynamics simulations. Nucleic Acids Res 47(13):6618–6631
Duan L, Liu X, Zhang JZH (2016) Interaction entropy: a new paradigm for highly efficient and reliable computation of protein-ligand binding free energy. J Am Chem Soc 138(17):5722–5728
Zhang Y, Ying JB, Hong JJ, Li FC, Fu TT, Yang FY, Zheng GX, Yao XJ, Lou Y, Qiu Y, Xue WW, Zhu F (2019) How does chirality determine the selective inhibition of histone deacetylase 6? A lesson from trichostatin A enantiomers based on molecular dynamics. ACS Chem Neurosci 10(5):2467–2480
Lin B, Zhang H, Zheng Q (2020) How do mutations affect the structural characteristics and substrate binding of CYP21A2? An investigation by molecular dynamics simulations. Phys Chem Chem Phys 22(16):8870–8877
Xue W, Wang P, Tu G, Yang F, Zheng G, Li X, Li X, Chen Y, Yao X, Zhu F (2018) Computational identification of the binding mechanism of a triple reuptake inhibitor amitifadine for the treatment of major depressive disorder. Phys Chem Chem Phys 20(9):6606–6616
Sun Z, He Q, Li X, Zhu Z (2020) SAMPL6 host–guest binding affinities and binding poses from spherical-coordinates-biased simulations. J Comput Aid Mol Des 34(5):589–600
Chen J, Liu X, Zhang S, Chen J, Sun H, Zhang L, Zhang Q (2020) Molecular mechanism with regard to the binding selectivity of inhibitors toward FABP5 and FABP7 explored by multiple short molecular dynamics simulations and free energy analyses. Phys Chem Chem Phys 22(4):2262–2275
He M-Y, Li W-K, Meiler J, Zheng Q-C, Zhang H-X (2019) Insight on mutation-induced resistance to anaplastic lymphoma kinase inhibitor ceritinib from molecular dynamics simulations. Biopolymers 110(2):e23257
Tu J, Song LT, Liu RR, Zhai HL, Wang J, Zhang XY (2019) Molecular inhibitory mechanism study on the potent inhibitor brigatinib against four crizotinib-resistant ALK mutations. J Cell Biochem 120(1):562–574
Kong X, Pan P, Sun H, Xia H, Wang X, Li Y, Hou T (2019) Drug discovery targeting anaplastic lymphoma kinase (ALK). J Med Chem 62(24):10927–10954
Pan P, Yu H, Liu Q, Kong X, Chen H, Chen J, Liu Q, Li D, Kang Y, Sun H, Zhou W, Tian S, Cui S, Zhu F, Li Y, Huang Y, Hou T (2017) Combating drug-resistant mutants of anaplastic lymphoma kinase with potent and selective type-I1/2 inhibitors by stabilizing unique DFG-shifted loop conformation. ACS Central Sci 3(11):1208–1220
Chen J, Wang J, Zhu W (2017) Mutation L1196M-induced conformational changes and the drug resistant mechanism of anaplastic lymphoma kinase studied by free energy perturbation and umbrella sampling. Phys Chem Chem Phys 19(44):30239–30248
He M-Y, Li W-K, Zheng Q-C, Zhang H-X (2018) Conformational transition of key structural features involved in activation of ALK induced by two neuroblastoma mutations and ATP binding: insight from accelerated molecular dynamics simulations. ACS Chem Neurosci 9(7):1783–1792
Xiao Z, Cong Y, Huang K, Zhong S, Zhang JZH, Duan L (2019) Drug-resistance mechanisms of three mutations in anaplastic lymphoma kinase against two inhibitors based on MM/PBSA combined with interaction entropy. Phys Chem Chem Phys 21(37):20951–20964
Miao Y, Feher VA, McCammon JA (2015) Gaussian accelerated molecular dynamics: unconstrained enhanced sampling and free energy calculation. J Chem Theory Comput 11(8):3584–3595
Miao Y, McCammon JA (2016) Graded activation and free energy landscapes of a muscarinic G-protein–coupled receptor. Proc Natl Acad Sci U S A 113(43):12162–12167
Wang J, Miao Y (2019) Mechanistic insights into specific G protein interactions with adenosine receptors. J Phys Chem B 123(30):6462–6473
Wang J, Alekseenko A, Kozakov D, Miao Y (2019) Improved modeling of peptide-protein binding through global docking and accelerated molecular dynamics simulations. Front Mol Biosci 6(112):112
Ricci CG, Chen JS, Miao Y, Jinek M, Doudna JA, McCammon JA, Palermo G (2019) Deciphering off-target effects in CRISPR-Cas9 through accelerated molecular dynamics. ACS Central Sci 5(4):651–662
An X, Bai Q, Bing Z, Liu H, Zhang Q, Liu H, Yao X (2020) Revealing the positive binding cooperativity mechanism between the orthosteric and the allosteric antagonists of CCR2 by metadynamics and Gaussian accelerated molecular dynamics simulations. ACS Chem Neurosci 11(4):628–637
Duan L, Gue X, Cong Y, Feng G, Li Y, Zhang JZH (2019) Accelerated molecular dynamics simulation for helical proteins folding in explicit water. Front Chem 7:540
Chen J, Wang W, Pang L, Zhu W (2020) Unveiling conformational dynamics changes of H-Ras induced by mutations based on accelerated molecular dynamics. Phys Chem Chem Phys 22(37):21238–21250
Hayward S, Kitao A, Gō N (1995) Harmonicity and anharmonicity in protein dynamics: a normal mode analysis and principal component analysis. Proteins Struct Funct Genet 23(2):177–186
Amadei A, Linssen ABM, Berendsen HJC (1993) Essential dynamics of proteins. Proteins Struct Funct Genet 17(4): 412–425.
Ichiye T, Karplus M (1991) Collective motions in proteins: a covariance analysis of atomic fluctuations in molecular dynamics and normal mode simulations. Proteins Struct Funct Genet 11(3):205–217
Levy RM, Srinivasan AR, Olson WK, McCammon JA (1984) Quasi-harmonic method for studying very low frequency modes in proteins. Biopolymers 23(6):1099–1112
Webb B, Sali A (2014) Comparative protein structure modeling using MODELLER. Current protocols in bioinformatics. Wiley, pp 2015.2016.2011–2015.2016.2032.
Li H, Robertson AD, Jensen JH (2005) Very fast empirical prediction and rationalization of protein pKa values. Proteins Struct Funct Genet 61(4):704–721
Bas DC, Rogers DM, Jensen JH (2008) Very fast prediction and rationalization of pKa values for protein–ligand complexes. Proteins Struct Funct Genet 73(3):765–783
Jakalian A, Jack DB, Bayly CI (2002) Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J Comput Chem 23(16):1623–1641
Jakalian A, Bush BL, Jack DB, Bayly CI (2000) Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J Comput Chem 21(2):132–146
Salomon-Ferrer R, Case DA, Walker RC (2013) An overview of the Amber biomolecular simulation package. WIREs Comput Mol Sci 3(2):198–210
Case DA, Cheatham TE III, Darden T, Gohlke H, Luo R, Merz KM Jr, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26(16):1668–1688
Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11(8):3696–3713
Song D, Luo R, Chen H-F (2017) The IDP-specific force field ff14IDPSFF improves the conformer sampling of intrinsically disordered proteins. J Chem Inf Model 57(5):1166–1178
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25(9):1157–1174
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935
Ȧqvist J (1990) Ion-water interaction potentials derived from free energy perturbation simulations. J Phys Chem B 94(21):8021–8024
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J Chem Phys 98(12):10089–10092
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103(19):8577–8593
Miao Y, Sinko W, Pierce L, Bucher D, Walker RC, McCammon JA (2014) Improved reweighting of accelerated molecular dynamics simulations for free energy calculation. J Chem Theory Comput 10(7):2677–2689
Ryckaert J-P, Ciccotti G, Berendsen HJC (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23(3):327–341
Izaguirre JA, Catarello DP, Wozniak JM, Skeel RD (2001) Langevin stabilization of molecular dynamics. J Chem Phys 114(5):2090–2098
Le Grand S, Götz AW, Walker RC (2013) SPFP: speed without compromise—a mixed precision model for GPU accelerated molecular dynamics simulations. Comput Phys Commun 184(2):374–380
Salomon-Ferrer R, Götz AW, Poole D, Le Grand S, Walker RC (2013) Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J Chem Theory Comput 9(9):3878–3888
Götz AW, Williamson MJ, Xu D, Poole D, Le Grand S, Walker RC (2012) Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized Born. J Chem Theory Comput 8(5):1542–1555
Roe DR, Cheatham TE (2013) PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J Chem Theory Comput 9(7):3084–3095
Chen J, Wang J, Yin B, Pang L, Wang W, Zhu W (2019) Molecular mechanism of binding selectivity of inhibitors toward BACE1 and BACE2 revealed by multiple short molecular dynamics simulations and free-energy predictions. ACS Chem Neurosci 10(10):4303–4318
Chen J, Yin B, Wang W, Sun H (2020) Effects of disulfide bonds on binding of inhibitors to β-amyloid cleaving enzyme 1 decoded by multiple replica accelerated molecular dynamics simulations. ACS Chem Neurosci 11(12):1811–1826
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Wang W, Kollman PA (2000) Free energy calculations on dimer stability of the HIV protease using molecular dynamics and a continuum solvent model11Edited by B Honig. J Mol Biol 303(4):567–582
Wang W, Kollman PA (2001) Computational study of protein specificity: The molecular basis of HIV-1 protease drug resistance. Proc Natl Acad Sci USA 98(26):14937–14942
Wang C, Greene DA, Xiao L, Qi R, Luo R (2018) Recent developments and applications of the MMPBSA method. Front Mol Biosci 4(87):87
Hu G, Li H, Xu S, Wang J (2020) Ligand binding mechanism and its relationship with conformational changes in Adenine Riboswitch. Int J Mol Sci 21(6): 1926.
Hou T, Wang J, Li Y, Wang W (2011) Assessing the performance of the molecular mechanics/Poisson Boltzmann surface area and molecular mechanics/generalized Born surface area methods. II. The accuracy of ranking poses generated from docking. J Comput Chem 32(5): 866–877.
Sun H, Li Y, Tian S, Xu L, Hou T (2014) Assessing the performance of MM/PBSA and MM/GBSA methods. 4. Accuracies of MM/PBSA and MM/GBSA methodologies evaluated by various simulation protocols using PDBbind data set. Phys Chem Chem Phys 16(31): 16719–16729.
Sun H, Li Y, Shen M, Tian S, Xu L, Pan P, Guan Y, Hou T (2014) Assessing the performance of MM/PBSA and MM/GBSA methods. 5. Improved docking performance using high solute dielectric constant MM/GBSA and MM/PBSA rescoring. Phys Chem Chem Phys 16(40): 22035–22045.
Miller BR, McGee TD, Swails JM, Homeyer N, Gohlke H, Roitberg AE (2012) MMPBSA.py: an efficient program for end-state free energy calculations. J Chem Theory Comput 8(9):3314–3321
Onufriev A, Bashford D, Case DA (2004) Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins Struct Funct Genet 55(2):383–394
Gohlke H, Kiel C, Case DA (2003) Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras–RalGDS complexes. J Mol Biol 330(4):891–913
Chen J, Wang X, Zhu T, Zhang Q, Zhang JZH (2015) A Comparative insight into amprenavir resistance of mutations V32I, G48V, I50V, I54V, and I84V in HIV-1 protease based on thermodynamic integration and MM-PBSA methods. J Chem Inf Model 55(9):1903–1913
Ding Y, Mei Y, Zhang JZH (2008) Quantum mechanical studies of residue-specific hydrophobic interactions in p53−MDM2 binding. J Phys Chem B 112(36):11396–11401
Acknowledgements
This work is supported the National Natural Science Foundation of China (11504206), Shandong Provincial Natural Science Foundation (ZR2017MA040), and the key research and development project of Shandong province (Nos. 2018GSF121014 and 2019GGX102050).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Wang, W., Sun, H. et al. Mutation-mediated influences on binding of anaplastic lymphoma kinase to crizotinib decoded by multiple replica Gaussian accelerated molecular dynamics. J Comput Aided Mol Des 34, 1289–1305 (2020). https://doi.org/10.1007/s10822-020-00355-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-020-00355-5