Abstract
Purpose
To utilize a novel mitochondrial function assay with pooled granulosa cells to determine whether mitochondrial function would differ by patient demographics and embryo development.
Methods
This was a prospective pilot study in a hospital-based assisted reproductive program and public university. Mitochondrial metabolic substrate utilization was assessed in pooled granulosa cells from 40 women undergoing in vitro fertilization during 2018 and 2019.
Results
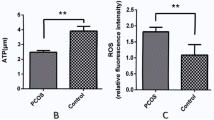
Assessment of mitochondrial substrate metabolism in pooled granulosa cells revealed higher citric acid, L-malic acid, and octanoyl-L-carnitine utilization with higher body mass index (BMI). Utilization of citric acid, cis-aconitic acid, D-alpha-keto-glutaric acid, L-glutamine, and alanine plus glycine was significantly lower as total dosage of FSH administered increased. Utilization of glycogen was significantly higher in patients with a higher percentage of fertilized oocytes. D-alpha-keto-glutaric acid utilization was significantly lower in patients with a higher percentage of good 8-cell embryos. L-glutamine utilization was significantly lower, with a higher percentage of blastocyst formation. Mitochondrial metabolic scores (MMS), which reflect overall mitochondrial activity of the granulosa pool, were significantly higher in patients with higher BMI and with greater numbers of mature oocytes retrieved. MMS in granulosa decreased as total FSH dose administered increased.
Conclusions
Granulosa cell utilization of substrates feeding into the citric acid cycle changed with total FSH dosage and BMI. Fertilization rate, 8-cell embryo quality, and blastocyst formation also associated with different energy substrate usage. Mitochondrial substrate utilization by granulosa cells from individual follicles could be further developed into a useful diagnostic tool.








Similar content being viewed by others
References
Margulis L. Archaeal-eubacterial mergers in the origin of Eukarya: phylogenetic classification of life. Proc Natl Acad Sci U S A. 1996;93(3):1071–6. https://doi.org/10.1073/pnas.93.3.1071.
Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond). 2010;7:7. doi:https://doi.org/10.1186/1743-7075-7-7.
Veatch JR, McMurray MA, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. 2009;137(7):1247–58. https://doi.org/10.1016/j.cell.2009.04.014.
Boucret L, JM CdlB, Moriniere C, Desquiret V, Ferre-L'Hotellier V, Descamps P et al. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015;30(7):1653–64. doi:dev114 [pii];https://doi.org/10.1093/humrep/dev114.
Pawlak P, Chabowska A, Malyszka N, Lechniak D. Mitochondria and mitochondrial DNA in porcine oocytes and cumulus cells--a search for developmental competence marker. Mitochondrion. 2016;27:48–55. https://doi.org/10.1016/j.mito.2015.12.008.
Collado-Fernandez E, Picton HM, Dumollard R. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 2012;56(10–12):799–808. https://doi.org/10.1387/ijdb.120140ec.
Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42. https://doi.org/10.1055/s-0028-1108008.
Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139(4):685–95. https://doi.org/10.1530/REP-09-0345.
Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229(3):353–61. https://doi.org/10.1002/jcp.24457.
Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16(10):715–25. doi:gaq031 [pii];https://doi.org/10.1093/molehr/gaq031.
Tsai HD, Hsieh YY, Hsieh JN, Chang CC, Yang CY, Yang JG, et al. Mitochondria DNA deletion and copy numbers of cumulus cells associated with in vitro fertilization outcomes. J Reprod Med. 2010;55(11–12):491–7.
Desquiret-Dumas V, Clement A, Seegers V, Boucret L, Ferre-L'Hotellier V, Bouet PE, et al. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod. 2017;32(3):607–14. https://doi.org/10.1093/humrep/dew341.
Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril. 2015;104(3):534–41 e1. https://doi.org/10.1016/j.fertnstert.2015.05.022.
Fragouli E, Wells D. Mitochondrial DNA assessment to determine oocyte and embryo viability. Semin Reprod Med. 2015;33(6):401–9. https://doi.org/10.1055/s-0035-1567821.
Ogino M, Tsubamoto H, Sakata K, Oohama N, Hayakawa H, Kojima T, et al. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J Assist Reprod Genet. 2016;33(3):367–71. https://doi.org/10.1007/s10815-015-0621-0.
Bochner BR, Gadzinski P, Panomitros E. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001;11(7):1246–55. https://doi.org/10.1101/gr.186501.
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, et al. Standardization of grading embryo morphology. J Assist Reprod Genet. 2010;27(8):437–9. https://doi.org/10.1007/s10815-010-9443-2.
Vanderstichel R, Dohoo I, Markham F. Applying a kinetic method to an indirect ELISA measuring Ostertagia ostertagi antibodies in milk. Can J Vet Res. 2015;79(3):180–3.
Bates D, Machler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi: https://doi.org/10.18637/jss.v067/i01.
Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(4):439–51. https://doi.org/10.1093/humupd/dmz011.
Hauck AK, Bernlohr DA. Oxidative stress and lipotoxicity. J Lipid Res. 2016;57(11):1976–86. https://doi.org/10.1194/jlr.R066597.
Gorshinova VK, Tsvirkun DV, Sukhanova IA, Tarasova NV, Volodina MA, Marey MV, et al. Cumulus cell mitochondrial activity in relation to body mass index in women undergoing assisted reproductive therapy. BBA Clin. 2017;7:141–6. https://doi.org/10.1016/j.bbacli.2017.03.005.
Abraham M, Collins CA, Flewelling S, Camazine M, Cahill A, Cade WT, et al. Mitochondrial inefficiency in infants born to overweight African-American mothers. Int J Obes. 2018;42(7):1306–16. https://doi.org/10.1038/s41366-018-0051-z.
Broekmans FJ. Individualization of FSH doses in assisted reproduction: facts and fiction. Front Endocrinol (Lausanne). 2019;10:181. doi:https://doi.org/10.3389/fendo.2019.00181.
Fedorcsak P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19(11):2523–8. https://doi.org/10.1093/humrep/deh485.
Borges E Jr, Zanetti BF, Setti AS, Braga DP, Figueira RCS, Iaconelli A Jr. FSH dose to stimulate different patient' ages: when less is more. JBRA Assist Reprod. 2017;21(4):336–42. https://doi.org/10.5935/1518-0557.20170058.
Saito N, Yamashita Y, Ono Y, Higuchi Y, Hayashi A, Yoshida Y, et al. Difference in mitochondrial gene expression in granulosa cells between recombinant FSH and hMG cycles under in vitro fertilization and transfer. Reprod Med Biol. 2013;12(3):99–104. https://doi.org/10.1007/s12522-013-0147-z.
Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28(9):773–83. https://doi.org/10.1007/s10815-011-9588-7.
May-Panloup P, Chretien MF, Jacques C, Vasseur C, Malthiery Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod. 2005;20(3):593–7. https://doi.org/10.1093/humrep/deh667.
Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–9. https://doi.org/10.1093/molehr/7.5.425.
Zhao SY, Qiao J, Chen YJ, Liu P, Li J, Yan J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril. 2010;94(1):261–7. doi:S0015–0282(09)00533–0 [pii];https://doi.org/10.1016/j.fertnstert.2009.03.014 .
Dumesic DA, Guedikian AA, Madrigal VK, Phan JD, Hill DL, Alvarez JP, et al. Cumulus cell mitochondrial resistance to stress in vitro predicts oocyte development during assisted reproduction. J Clin Endocrinol Metab. 2016;101(5):2235–45. https://doi.org/10.1210/jc.2016-1464.
Brogan RS, MacGibeny M, Mix S, Thompson C, Puttabyatappa M, VandeVoort CA, et al. Dynamics of intra-follicular glucose during luteinization of macaque ovarian follicles. Mol Cell Endocrinol. 2011;332(1–2):189–95. https://doi.org/10.1016/j.mce.2010.10.011.
Taugourdeau A, Desquiret-Dumas V, Hamel JF, Chupin S, Boucret L, Ferre-L'Hotellier V, et al. The mitochondrial DNA content of cumulus cells may help predict embryo implantation. J Assist Reprod Genet. 2019;36(2):223–8. https://doi.org/10.1007/s10815-018-1348-5.
Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30(10):1367–75. https://doi.org/10.1007/s10815-013-0062-6.
Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85(3):584–91. https://doi.org/10.1016/j.fertnstert.2005.09.017.
Kordus RJ, LaVoie HA. Granulosa cell biomarkers to predict pregnancy in ART: pieces to solve the puzzle. Reproduction. 2017;153(2):R69-R83. doi:REP-16-0500 [pii];https://doi.org/10.1530/REP-16-0500.
Bochner BR, Siri M, Huang RH, Noble S, Lei XH, Clemons PA, et al. Assay of the multiple energy-producing pathways of mammalian cells. PLoS One. 2011;6(3):e18147. https://doi.org/10.1371/journal.pone.0018147.
Kuzniewska B, Cysewski D, Wasilewski M, Sakowska P, Milek J, Kulinski TM et al. Mitochondrial protein biogenesis in the synapse is supported by local translation. EMBO Rep. 2020:e48882. doi:https://doi.org/10.15252/embr.201948882.
Funding
Supported by an ASPIRE-I grant from the University of South Carolina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this study.
Ethical approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and in its later amendments or comparable ethical standards.” This study was approved by the Greenville Health System (IRB number: Pro00072006) and the University of South Carolina Institution Review Board (IRB registration number: 00000240).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary data are available at the Journal of Assisted Reproduction and Genetics online.
ESM 1
Plot of granulosa cell number per well versus the difference in absorbance between the positive and negative controls for the Mitoplate S-1 assays confirm the linear relationship. Data include n = 45 patients including those used in optimization experiments. Patients showing no absorbance difference between the controls at approximately 6500 cells or less were not included. (PDF 230 kb)
ESM 2
Substrates that demonstrated no significant mitochondrial utilization differences based on body mass index (BMI). To determine the differences in mitochondrial utilization, substrates were compared using a multiple linear regression model using Box-Cox transformation between BMI groups. BMI was divided into 2 groups with the higher group being patients with a BMI of ≥ to 26 kg/m2 (n = 20) and the lower group being patients with a BMI of <26 kg/m2 (n = 20). Mitochondrial utilization of cis-aconitic acid (P < 0.10), D,L-beta-hydroxy-butyric acid (P < 0.10), and pyruvic acid with L-malic acid 100 μM (P < 0.10) trended to be higher in pooled GCs of patients with higher BMI, after adjusting for the other factors in the model. No other substrates showed any mitochondrial utilization differences. Following the Box-Cox transformation of the data and observing the Q-Q plots, the most outlying observations have been removed to ensure the normality of the response variables. Data are presented as the median (line inside box), first and third quartile (bottom and top of the box), and highest and lowest data points (top and bottom of whiskers). (PDF 8162 kb)
ESM 3
Substrates that demonstrated no significant mitochondrial utilization differences based number (A) and percentage (B) of mature oocytes retrieved. To determine the differences in mitochondrial utilization, substrates were compared using a multiple linear regression model using Box-Cox transformation. No substrates in pooled GCs of patients demonstrated any significant mitochondrial utilization differences after adjusting for the other factors in the model. Following the Box-Cox transformation of the data and observing the Q-Q plots, the most outlying observations have been removed to ensure the normality of the response variables. Data are presented as a scatter plot of the individual patient values lambda transformed for normalization showing the trend line and the 95% confidence interval (PDF 8525 kb)
ESM 4
Substrates that demonstrated no significant mitochondrial utilization differences based on total FSH dose administered. To determine the differences in mitochondrial utilization, substrates were compared using a multiple linear regression model using Box-Cox transformation. Mitochondrial utilization of D,L-isocitric acid (P < 0.10), D,L-beta-hydroxy-butyric acid (P < 0.10), L-glutamic acid (P < 0.10), acetyl-L-carnitine (P < 0.10), pyruvic acid with L-malic acid 100 μM (P < 0.10) trended to be lower in pooled GCs of patients with higher administered FSH dosages, after adjusting for the other factors in the model. No other substrates showed any mitochondrial utilization differences. Following the Box-Cox transformation of the data and observing the Q-Q plots, the most outlying observations have been removed to ensure the normality of the response variables. Data are presented as a scatter plot of the individual patient values lambda transformed for normalization showing the trend line and the 95% confidence interval (PDF 6449 kb)
ESM 5
Substrates that demonstrated no significant mitochondrial utilization differences based fertilization percentage. To determine the differences in mitochondrial utilization, substrates were compared using a multiple linear regression model using Box-Cox transformation. The two groups were delineated by higher fertilization percentage ≥ 80% (n = 22), and lower fertilization percentage < 80% (n = 18). Following the Box-Cox transformation of the data and observing the Q-Q plots, the most outlying observations have been removed to ensure the normality of the response variables. Data are presented as the median (line inside box), first and third quartile (bottom and top of the box), and highest and lowest data points (top and bottom of whiskers) (PDF 7626 kb)
ESM 6
Substrates that demonstrated no significant mitochondrial utilization differences based on good 8-cell embryo development. To determine the differences in mitochondrial utilization, substrates were compared using a multiple linear regression model using Box-Cox transformation. The two groups were delineated by higher good 8-cell development >55% (n = 22) and lower good 8-cell development ≤55% (n = 18). Mitochondrial utilization of L-serine (P < 0.10) trended to be lower in GCs of patients with a higher percentage of good quality 8-cell embryos. In a backward selected best fit model using only the percentage of good 8-cell embryos and percentage of blastocysts, L-glutamine (P < 0.10) trended to be lower in pooled GCs from patients with a higher percentage of good quality 8-cell embryos. No other substrates showed significant differences. Following the Box-Cox transformation of the data and observing the Q-Q plots, the most outlying observations have been removed to ensure the normality of the response variables. Data are presented as the median (line inside box), first and third quartile (bottom and top of the box), and highest and lowest data points (top and bottom of whiskers) (PDF 7199 kb)
ESM 7
Substrates that demonstrated no significant mitochondrial utilization differences based on percent blastocyst formation. To determine the differences in mitochondrial utilization, substrates were compared using a multiple linear regression model using Box-Cox transformation. The two groups were delineated by higher percentage of blastocyst formation >55% (n = 17) and lower percentage of blastocyst formation ≤55% (n = 22). Granulosa mitochondrial utilization of D-Gluconate-6-phosphate (P < 0.10) and D,L-isocitric acid (P < 0.10) trended to be lower in patients with a higher percentage of blastocyst formation, after adjusting for the other factors in the model. No other substrates demonstrated significant differences. Following the Box-Cox transformation of the data and observing the Q-Q plots, the most outlying observations have been removed to ensure the normality of the response variables. Data are presented as the median (line inside box), first and third quartile (bottom and top of the box), and highest and lowest data points (top and bottom of whiskers) (PDF 7111 kb)
ESM 8
Pooled granulosa cell mitochondrial DNA (mtDNA) content had no significant association with patient demographics nor oocyte outcomes. Correlation analysis showed mtDNA was similar between all groups. For categorical groups, data are presented as the median (line inside box), first and third quartile (bottom and top of the box), and highest and lowest data points (top and bottom of whiskers). For continuous variables, data are presented as a scatter plot of the individual patient values lambda transformed for normalization showing the trend line and the 95% confidence interval. 8-cells = 8-cell embryos, AFC = antral follicle count, AMH = anti-Mullerian hormone, ANT = antagonist stimulation, BMI = body mass index, FSH = follicle stimulating hormone, HMG = human menopausal gonadotropin, LL = long luteal stimulation, STIM = stimulation type (PDF 3804 kb)
Rights and permissions
About this article
Cite this article
Kordus, R.J., Hossain, A., Malter, H.E. et al. Mitochondrial metabolic substrate utilization in granulosa cells reflects body mass index and total follicle stimulating hormone dosage in in vitro fertilization patients. J Assist Reprod Genet 37, 2743–2756 (2020). https://doi.org/10.1007/s10815-020-01946-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01946-9