Abstract
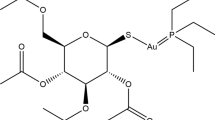
More than 30 years ago, auranofin was developed for the treatment of rheumatoid arthritis as a substitution for the injectable gold compounds aurothiomalate and aurothioglucose. Both the ease of oral administration over intramuscular injections and more potent anti-inflammatory effects in vitro made auranofin seem like an excellent substitute for the traditional injectable gold compounds. Despite efficacy in the treatment of both rheumatoid arthritis and psoriasis, currently, auranofin is seldom used as a treatment for patients with rheumatoid arthritis as more novel anti-rheumatic medications have become available. Despite the decline in its clinical applications, research on auranofin has continued as it shows promise in the treatment of several different diseases. In recent years, advances in technology have allowed researchers to use molecular techniques to identify novel mechanisms of action of auranofin. Additionally, researchers are discovering potential new applications of auranofin. Dual inhibition of inflammatory pathways and thiol redox enzymes by auranofin makes it a new candidate for cancer therapy and treating microbial infections. This review will summarize recently obtained data on the mechanisms of action of auranofin, and potential new applications of auranofin in the treatment of various diseases, including several types of leukaemia, carcinomas, and parasitic, bacterial, and viral infections.


Similar content being viewed by others
References
Angelucci F, Sayed AA, Williams DL, Boumis G, Brunori M, Dimastrogiovanni D, Miele AE, Pauly F, Bellelli A (2009) Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J Biol Chem 284:28977–28985
Arnason BG (1999) Immunologic therapy of multiple sclerosis. Annu Rev Med 50:291–302
Ashino T, Sugiuchi J, Uehara J, Naito-Yamamoto Y, Kenmotsu S, Iwakura Y, Shioda S, Numazawa S, Yoshida T (2011) Auranofin protects against cocaine-induced hepatic injury through induction of heme oxygenase-1. J Toxicol Sci 36:635–643
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Berners-Price SJ, Filipovska A (2011) Gold compounds as therapeutic agents for human diseases. Metallomics 3:863–873
Bonilla M, Denicola A, Novoselov SV, Turanov AA, Protasio A, Izmendi D, Gladyshev VN, Salinas G (2008) Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin glutathione reductase and dependent on selenium and glutathione. J Biol Chem 283:17898–17907
Brown NS, Bicknell R (2001) Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 3:323–327
Brown KK, Cox AG, Hampton MB (2010) Mitochondrial respiratory chain involvement in peroxiredoxin 3 oxidation by phenethyl isothiocyanate and auranofin. FEBS Lett 584:1257–1262
Caroli A, Simeoni S, Lepore R, Tramontano A, Via A (2012) Investigation of a potential mechanism for the inhibition of SmTGR by Auranofin and its implications for Plasmodium falciparum inhibition. Biochem Biophys Res Commun 417:576–581
Champion GD, Graham GG, Ziegler JB (1990) The gold complexes. Baillieres Clin Rheumatol 4:491–534
Chiellini C, Casini A, Cochet O, Gabbiani C, Ailhaud G, Dani C, Messori L, Amri EZ (2008) The influence of auranofin, a clinically established antiarthritic gold drug, on bone metabolism: analysis of its effects on human multipotent adipose-derived stem cells, taken as a model. Chem Biodivers 5:1513–1520
Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP (2009) HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900
Cox AG, Brown KK, Arner ES, Hampton MB (2008) The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem Pharmacol 76:1097–1109
Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, Garcia-Rivera G, Orozco E, Martinez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, Mckerrow JH, Reed SL (2012) A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med 18:956–960
Fauci AS (1996) Host factors and the pathogenesis of HIV-induced disease. Nature 384:529–534
Fonteh PN, Keter FK, Meyer D (2010) HIV therapeutic possibilities of gold compounds. Biometals 23:185–196
Frears ER, Zhang Z, Blake DR, O’connell JP, Winyard PG (1996) Inactivation of tissue inhibitor of metalloproteinase-1 by peroxynitrite. FEBS Lett 381:21–24
Glennas A, Kvien TK, Andrup O, Clarke-Jenssen O, Karstensen B, Brodin U (1997) Auranofin is safe and superior to placebo in elderly-onset rheumatoid arthritis. Br J Rheumatol 36:870–877
Greten FR, Karin M (2004) The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett 206:193–199
Griffioen AW, Molema G (2000) Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 52:237–268
Han S, Kim K, Kim H, Kwon J, Lee YH, Lee CK, Song Y, Lee SJ, Ha N (2008) Auranofin inhibits overproduction of pro-inflammatory cytokines, cyclooxygenase expression and PGE2 production in macrophages. Arch Pharm Res 31:67–74
Hileman EO, Liu J, Albitar M, Keating MJ, Huang P (2004) Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol 53:209–219
Hill KE, Mccollum GW, Boeglin ME, Burk RF (1997) Thioredoxin reductase activity is decreased by selenium deficiency. Biochem Biophys Res Commun 234:293–295
Ichimura K, Pearson DM, Kocialkowski S, Backlund LM, Chan R, Jones DT, Collins VP (2009) IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neurooncology 11:341–347
Jackson-Rosario S, Self WT (2009) Inhibition of selenium metabolism in the oral pathogen Treponema denticola. J Bacteriol 191:4035–4040
Jackson-Rosario S, Cowart D, Myers A, Tarrien R, Levine RL, Scott RA, Self WT (2009) Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au–Se adduct. J Biol Inorg Chem 14:507–519
Jeon KI, Jeong JY, Jue DM (2000) Thiol-reactive metal compounds inhibit NF-kappa B activation by blocking I kappa B kinase. J Immunol 164:5981–5989
Jones JS (1998) Life in the 21st century: a vision for all. S Afr Med J 88:674
Kean WF (1990) Intramuscular versus oral gold therapy. Baillieres Clin Rheumatol 4:219–246
Kean WF, Kean IR (2008) Clinical pharmacology of gold. Inflammopharmacology 16:112–125
Kean WF, Forestier F, Kassam Y, Buchanan WW, Rooney PJ (1985) The history of gold therapy in rheumatoid disease. Semin Arthritis Rheu 14:180–186
Kean WF, Hart L, Buchanan WW (1997) Auranofin. Br J Rheumatol 36:560–572
Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G (2006) TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res 66:3859–3868
Kim IS, Jin JY, Lee IH, Park SJ (2004) Auranofin induces apoptosis and when combined with retinoic acid enhances differentiation of acute promyelocytic leukaemia cells in vitro. Br J Pharmacol 142:749–755
Kim NH, Lee MY, Park SJ, Choi JS, Oh MK, Kim IS (2007) Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology 122:607–614
Kim NH, Oh MK, Park HJ, Kim IS (2010) Auranofin, a gold(I)-containing antirheumatic compound, activates Keap1/Nrf2 signaling via Rac1/iNOS signal and mitogen-activated protein kinase activation. J Pharmacol Sci 113:246–254
Korherr C, Gille H, Schafer R, Koenig-Hoffmann K, Dixelius J, Egland KA, Pastan I, Brinkmann U (2006) Identification of proangiogenic genes and pathways by high-throughput functional genomics: TBK1 and the IRF3 pathway. Proc Natl Acad Sci USA 103:4240–4245
Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Arner ES, Williams DL (2007) Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med 4:e206
Lewis MG, Dafonseca S, Chomont N, Palamara AT, Tardugno M, Mai A, Collins M, Wagner WL, Yalley-Ogunro J, Greenhouse J, Chirullo B, Norelli S, Garaci E, Savarino A (2011) Gold drug auranofin restricts the viral reservoir in the monkey AIDS model and induces containment of viral load following ART suspension. AIDS 25:1347–1356
Lo Vecchio A, Zacur GM (2012) Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol 28:1–9
Mancek-Keber M, Gradisar H, Inigo Pestana M, Martinez De Tejada G, Jerala R (2009) Free thiol group of MD-2 as the target for inhibition of the lipopolysaccharide-induced cell activation. J Biol Chem 284:19493–19500
Martinez-Gonzalez JJ, Guevara-Flores A, Alvarez G, Rendon-Gomez JL, Del Arenal IP (2010) In vitro killing action of auranofin on Taenia crassiceps metacestode (cysticerci) and inactivation of thioredoxin-glutathione reductase (TGR). Parasitol Res 107:227–231
Marzano C, Gandin V, Folda A, Scutari G, Bindoli A, Rigobello MP (2007) Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Rad Biol Med 42:872–881
Mckeage MJ, Berners-Price SJ, Galettis P, Bowen RJ, Brouwer W, Ding L, Zhuang L, Baguley BC (2000) Role of lipophilicity in determining cellular uptake and antitumour activity of gold phosphine complexes. Cancer Chemother Pharmacol 46:343–350
Moayeri M, Crown D, Dorward DW, Gardner D, Ward JM, Li Y, Cui X, Eichacker P, Leppla SH (2009) The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS). PLoS Pathog 5:e1000456
Nakaya A, Sagawa M, Muto A, Uchida H, Ikeda Y, Kizaki M (2011) The gold compound auranofin induces apoptosis of human multiple myeloma cells through both down-regulation of STAT3 and inhibition of NF-kappaB activity. Leuk Res 35:243–249
Newman ZL, Sirianni N, Mawhinney C, Lee MS, Leppla SH, Moayeri M, Johansen LM (2011) Auranofin protects against anthrax lethal toxin-induced activation of the Nlrp1b inflammasome. Antimicrob Agents Chemother 55:1028–1035
Omata Y, Lewis JB, Lockwood PE, Tseng WY, Messer RL, Bouillaguet S, Wataha JC (2006) Gold-induced reactive oxygen species (ROS) do not mediate suppression of monocytic mitochondrial or secretory function. Toxicol In Vitro 20:625–633
Otterbein LE, Soares MP, Yamashita K, Bach FH (2003) Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24:449–455
Papp KA, Shear NH (1991) Systemic gold therapy. Clin Dermatol 9:535–551
Park SJ, Kim IS (2005) The role of p38 MAPK activation in auranofin-induced apoptosis of human promyelocytic leukaemia HL-60 cells. Br J Pharmacol 146:506–513
Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, Eun HC, Chung JH (2004) Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J Invest Dermatol 123:1012–1019
Park SJ, Lee AN, Youn HS (2010) TBK1-targeted suppression of TRIF-dependent signaling pathway of toll-like receptor 3 by auranofin. Arch Pharm Res 33:939–945
Rabasseda X (2011) A report from the XVIII International AIDS Conference. (July 18–23, 2010-Vienna, Austria). Drugs Today (Barc) 46:945–957
Sannella AR, Casini A, Gabbiani C, Messori L, Bilia AR, Vincieri FF, Majori G, Severini C (2008) New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: mechanistic and pharmacological implications. FEBS Lett 582:844–847
Saura R, Matsubara T, Mizuno K (1994) Inhibition of neovascularization in vivo by gold compounds. Rheumatol Int 14:1–7
Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 22:203–208
Serhan CN, Drazen JM (1997) Antiinflammatory potential of lipoxygenase-derived eicosanoids: a molecular switch at 5 and 15 positions? J Clin Invest 99:1147–1148
Shabani F, Mcneil J, Tippett L (1998) The oxidative inactivation of tissue inhibitor of metalloproteinase-1 (TIMP-1) by hypochlorous acid (HOCI) is suppressed by anti-rheumatic drugs. Free Radic Res 28:115–123
Shapiro DL, Masci JR (1996) Treatment of HIV associated psoriatic arthritis with oral gold. J Rheumatol 23:1818–1820
Simonson LG, Goodman CH, Bial JJ, Morton HE (1988) Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun 56:726–728
Stanley BA, Sivakumaran V, Shi S, Mcdonald I, Lloyd D, Watson WH, Aon MA, Paolocci N (2011) Thioredoxin reductase-2 is essential for keeping low levels of H(2)O(2) emission from isolated heart mitochondria. J Biol Chem 286:33669–33677
Stern I, Wataha JC, Lewis JB, Messer RL, Lockwood PE, Tseng WY (2005) Anti-rheumatic gold compounds as sublethal modulators of monocytic LPS-induced cytokine secretion. Toxicol In Vitro 19:365–371
Talbot S, Nelson R, Self WT (2008) Arsenic trioxide and auranofin inhibit selenoprotein synthesis: implications for chemotherapy for acute promyelocytic leukaemia. Br J Pharmacol 154:940–948
Tepperman K, Finer R, Donovan S, Elder RC, Doi J, Ratliff D, Ng K (1984) Intestinal uptake and metabolism of auranofin, a new oral gold-based antiarthritis drug. Science 225:430–432
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Van Oosten BW, Lai M, Barkhof F, Miller DH, Moseley IF, Thompson AJ, Hodgkinson S, Polman CH (1996) A phase II trial of anti-CD4 antibodies in the treatment of multiple sclerosis. Mult Scler 1:339–342
Walz DT, Dimartino MJ, Griswold DE, Intoccia AP, Flanagan TL (1983) Biologic actions and pharmaco kinetic studies of auranofin. Am J Med 75:90–108
Winzeler EA (2008) Malaria research in the post-genomic era. Nature 455:751–756
Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S (1999) Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103:129–135
Yamada R, Sano H, Hla T, Hashiramoto A, Fukui W, Miyazaki S, Kohno M, Tsubouchi Y, Kusaka Y, Kondo M (1999) Auranofin inhibits interleukin-1beta-induced transcript of cyclooxygenase-2 on cultured human synoviocytes. Eur J Pharmacol 385:71–79
Yamamoto Y, Gaynor RB (2001) Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Investig 107:135–142
Yamashita M, Niki H, Yamada M, Watanabe-Kobayashi M, Mue S, Ohuchi K (1997) Dual effects of auranofin on prostaglandin E2 production by rat peritoneal macrophages. Eur J Pharmacol 325:221–227
Youn HS, Lee JY, Saitoh SI, Miyake K, Hwang DH (2006) Auranofin, as an anti-rheumatic gold compound, suppresses LPS-induced homodimerization of TLR4. Bioch Biophys Res Comm 350:866–871
Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH (1997) Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA 94:14848–14853
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Jack Brown and Family Alzheimer’s Disease Research Foundation. We would like to thank Ms. N. Gill for help with preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madeira, J.M., Gibson, D.L., Kean, W.F. et al. The biological activity of auranofin: implications for novel treatment of diseases. Inflammopharmacol 20, 297–306 (2012). https://doi.org/10.1007/s10787-012-0149-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-012-0149-1