Abstract
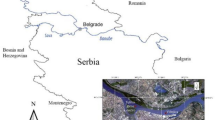
In mammals, it has been shown that halomethanes (HM) are bioactivated by enzymes such as CYP 2E1 and the theta isoform of GST to produce reactive metabolites. However, in fish, little information is available, although HM can form autochthonously in aquatic environments. This study assessed the effect of HM in dusky splitfin (Goodea gracilis) from three lakes of the Valley of Mexico by analysing specific HM biomarkers as well as a broad range of biomarkers. The concentration of HM was a function of its half-life (higher in deep waters), while its precursors and solar radiation are secondary factors that determine its concentration. The kidney showed higher basal metabolism than the liver, probably because of its function as a haematopoietic and filtration organ. Using integrated biological response version 2 (IBRv2), it was found that the hepatic and renal O2· content is a pro-oxidant force capable of inducing oxidative stress (ROOH, TBARS and RC=O). Early damage was found to be dependent on low concentrations of HM in Major Lake, whereas late damage was observed in fish exposed to higher concentrations of HM in Zumpango Lake and Ancient Lake. The activities of enzymes involved in antioxidant defence seemed to be inefficient. The quantitative assessment of biomarkers (ANOVA) and the estimate of parameter A obtained from IBRv2 provided different information. However, the data support the greater predictive power of IBRv2, but it requires a series of interrelated biomarkers to infer these possibilities. G. gracilis presents marked patterns of adaptation, which are dependant on the HM concentrations in environmental mixtures, although the response is complex and many toxicants could induce similar responses.







Similar content being viewed by others
References
ATSDR Agency for Toxic Substances and Disease Registry (1999) Diclorobromometano (Bromodichloromethane) ToxFacs
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Tox Chem 21(6):1316–1322
Bindokas VP, Jordán J, Lee CC, Miller RJ (1996) Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16:1324–1336
Borg DC, Schaich KM (1984) Cytotoxicity from coupled redox cycling of oxidizing xenobiotics and metal. Israel J Chem 24:38–53
Boryslawskyj M, Garrood AC, Pearson JT, Woodhead D (1998) Elevation of glutathione-S-transferase activity as a stress response to organochlorine compounds, in the freshwater mussel, Sphaerium corneum. Mar Environ Res 24:101–104
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Meth Enzymol 52:302–310
Cannady EA, Dyer CA, Christian PJ, Sipes G, Hoyer PB (2003) Expression and activity of cytochromes P4502E1, 2A, and 2B in the mouse ovary: the effects of 4-viylcyclohexane and its diepoxide metabolite. Toxicol Sci 73:423–430
Christof O, Seifert R, Michaelis W (2002) Volatile halogenated organic compounds in European Estuaries. Biogeochemistry 59:143–160
Davidson AJ, Zon LI (2004) The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23:7233–7246
Dekant W, Vamvakas S (1993) Glutathione-dependent bioactivation of xenobiotics. Xenobiotica 23(8):373–387
Elshorbagy W, Abdulkarim M (2006) Chlorination byproducts in drinking water produced from thermal desalination in United Arab Emirates. Environ Monit Assess 123(1–3):313–331
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15(6):1583–1606
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. In: Storey KB (ed) Functional metabolism: regulation and adaptation. Wiley-Liss, Inc., Hoboken, New Jersey, pp 319–351
Hodgson EK, Fridovich I (1975) The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry 14(24):5294–5299
Hua B, Veum K, Yang J, Jones J, Deung B (2010) Parallel factor analysis of fluorescence EEM spectra to identify THM precursors in lake waters. Environ Monit Assess 161(1–4):71–81
Iriarte-Velasco U, Álvarez-Uriarte JI, González-Velasco JR (2006) Kinetics of chloroform formation fron humic and fulvic acid chlorination. J Environ Sci Health A41:1495–1508
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Jiang ZY, Woollard AC, Wolff SP (1991) Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 26(10):853–856
Kitlar T, Döring F, Kinne RKH, Deutscher J, Diedrich DF, Frank R, Wallmeier H (1994) Interaction of phlorizin, a potent inhibitor of the Na +/d-glucose cotransporter, with the NADPH-binding site of mammalian catalases. Protein Sci 3(4):696–700
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5761
Kortmann RW, Rich PH (1994) Lake ecosystem energetics: the missing management link. Lake Reserv Manage 8:77–97
Le Cren E (1951) The length-weight relation and seasonal cycle in gonad weight and condition in the perch Perca fluviatilis. J Anim Ecol 20(2):201–219
Lei XG, Evenson JK, Thompson KM, Sunde RA (1995) Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J Nutr 125(6):1438–1446
Levine RL, Williams JA, Stadtman EP, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Matkovics B, Novák R, Hanh HD, Szabó L, Varga SZI (1977) A comparative study on some more important experimental animal peroxide metabolism enzymes. Comp Biochem Physiol, 56B 56(4):397–402
Mazari-Hiriart M, Ponce-de-León S, López-Vidal Y, Islas-Macías P, Amieva-Fernández RI, Quiñones-Falconi F (2008) Microbiological implications of periurban agriculture and water reuse in Mexico City. PLoS ONE 3(5):e2305
Melnick RL, Kohn MC, Dunnick JK, Leininger JR (1998) Regenerative hyperplasia is not required for liver tumor induction in female B6C3F1 mice exposed to trihalomethanes. Toxicol Appl Pharmacol 148(1):137–147
Misra HP, Fridovich I (1972) The role of superoxide dismutase anion in the autooxidation of ephinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Porubsky PR, Meneely KM, Scott EE (2008) Structures of human cytochrome P-450 2E1, insights into the binding of inhibitors and both small molecular weight and fatty acid substrates. J Biol Chem 283(48):33698–33707
Radi R, Turrens JF, Chang LY, Bush KM, Crapo JD, Freeman BA (1991) Detection of catalase in rat heart mitochondria. J Biol Chem 266:22028–22034
SAGARPA (2001) Norma Oficial Mexicana NOM-062-ZOO-1999 Especificaciones técnicas para la producción, cuidado y el uso de animales de laboratorio. Diario Oficial de la Federación. Segunda Sección, México, pp 1–57
Salameh E, Alawi M, Batarseh M, Jiries A (2002) Determination of trihalomethanes and the ionic composition of groundwater at Amman City, Jordan. Hydrogeol J 10:332–339
Sanchez W, Burgeot T, Porcher JM (2012) A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ Sci Pollut Res Int 20(5):2721–2725
Sapone A, Gustavino B, Monfrinotti M, Canastro D, Brócoli M, Pozzetti L, Affatato A, Valgimigli L, Forti GC, Pedulli GF, Biagi GL, Andel-Rahman SZ, Paolini M (2007) Perturbation of cytochrome P450, generation of oxidative stress and induction of DNA damage in Cyprinus carpio exposed in situ to potable surface water. Mut Res 626(1–2):143–154
Senem-Uyguner C, Hellriegel C, Otto W, Larive CK (2004) Characterization of humic substances: implications for trihalomethane formation. Analyt Bioanalyt Chem 378:1579–1586
Stachura DL, Reyes JR, Bartunek P, Paw BH, Zon LI, Traver D (2009) Zebrafish kidney stromal cell lines support multilineage hematopoiesis. Blood 114(2):279–289
Tanabe S, Minh TB (2010) Dioxins and organohalogen contaminants in the Asia-Pacific region. Ecotoxicology 19(3):463–478
Teuschler LK, Gennings C, Stiteler WM, Hertberg RC, Colman JT, Thiyagarajah A, Lipscomb JC, Hartley WR, Simmons JE (2000) A multiple-purpose design approach to the evaluation of risks from mixtures of disinfection by-products. Drug Chem Toxicol 23(1):307–321
Vega-López A, Jiménez-Orozco FA, García-Latorre E, Domínguez-López ML (2008) Oxidative stress response in an endangered goodeid fish (Girardinichthys viviparus) by exposure to water from its extant localities. Ecotoxicol Environ Saf 71(1):94–103
Vega-López A, Jiménez-Orozco FA, Jiménez-Zamudio LA, García-Latorre E, Domínguez-López ML (2009) Phase I enzyme induction in Girardinichthys viviparus, an endangered goodeid fish, exposed to water from native localities enriched with polychlorinated biphenyls. Arch Environ Contam Toxicol 57(3):561–570
Vega-López A, Carrillo-Morales CI, Olivares-Rubio HF, Domínguez-López ML, García-Latorre E (2012) Evidence of bioactivation of halomethanes and its relation to oxidative stress response in Chirostoma riojai, an endangered fish from a polluted lake in Mexico. Arch Environ Contam Toxicol 62(3):479–493
Vega-López A, Ayala-López G, Posadas-Espadas BP, Olivares-Rubio HF, Dzul-Caamal R (2013) Relations of oxidative stress in freshwater phytoplankton with heavy metals and polycyclic aromatic hydrocarbons. Comp Biochem Physiol A Mol Integr Physiol. doi:10.1016/j.cbpa.2013.01.026. (Epub ahead of print)
Warholm M, Alexandre A-K, Högberg J, Sigvardsson K, Rannung A (1994) Polymorphic distribution of gluthatione transferase activity with methyl chloride in human blood. Pharmacogenetics 4:307–311
Acknowledgments
This study was financed by CONACyT-ICyTDF and SIP-IPN. H. F. Olivares-Rubio is a M.Sc. student with a scholarship from CONACyT. M. L. Martínez-Torres is a B.Sc. student with a scholarship from CONACyT. M. L. Domínguez-López, E. García-Latorre and A. Vega-López are fellows of Estímulos al Desempeño en Investigación and Comisión y Fomento de Actividades Académicas (Instituto Politécnico Nacional) and Sistema Nacional de Investigadores (SNI, CONACyT, México).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olivares-Rubio, H.F., Martínez-Torres, M.L., Domínguez-López, M.L. et al. Pro-oxidant and antioxidant responses in the liver and kidney of wild Goodea gracilis and their relation with halomethanes bioactivation. Fish Physiol Biochem 39, 1603–1617 (2013). https://doi.org/10.1007/s10695-013-9812-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9812-8