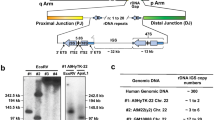
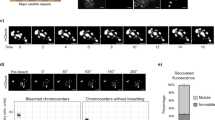
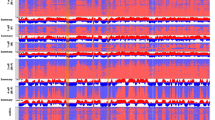
Abstract
Ribosomal DNA (rDNA) gene codes for 18S, 5.8S, and 28S rRNA form tandem repetitive clusters, which occupy distinct chromosomal loci called nucleolar organizer regions (NORs). The number and position of NORs on chromosomes are genetic characteristics of the species although within a cell, the NOR sizes can significantly vary due to loss or multiplication of rDNA copies. In the current study, we used mouse L929 fibroblasts, the aneuploid cells which differ in the FISH- and Ag-NOR numbers, to examine whether the parental NOR variability is inherited in clones. By statistical analysis, we showed that the cloned fibroblasts were able to restore the NOR numerical characteristics of the parental cells after long-term culturing. These results support the idea that mammalian cells may have mechanisms which control the number and activity of NORs at the population level. In L929 fibroblasts, we also regularly observed laterally asymmetry of FISH-NORs that evidenced in an unequal distribution of the mother rDNA copies between the daughter cells in mitosis.







Similar content being viewed by others
Abbreviations
- NOR:
-
Nucleolar organizer region
- rDNA:
-
Ribosomal DNA
- rRNA:
-
Ribosomal RNA
- 5′ETS:
-
5′ External transcribed spacer
- ITS1 and ITS2:
-
The first and second internal transcribed spacers
- FISH:
-
Fluorescence in situ hybridization
- FISH-NOR:
-
NOR hybridized with rDNA-FISH probes
- Ag-NOR:
-
Silver-positive NOR
References
Andreeva EV, Petrov IP, Semenova EG (1987) Changes in the karyotype and surface properties of L cells during the adaptation of a suspension culture to monolayer growth. Tsitologiia 29:684–688
Britton-Davidian J, Cazaux B, Catalan J (2012) Chromosomal dynamics of nucleolar organizer regions (NORs) in the house mouse: micro-evolutionary insights. Heredity 108:68–74. https://doi.org/10.1038/hdy.2011.105
Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI (2007) The multifunctional nucleolus. Nat Rev Mol Cell Biol 8:574–585
Davisson MT, Akeson EC (1993) Recombination suppression by heterozygous Robertsonian chromosomes in the mouse. Genetics 133(3):649–667
Gibbons JG, Branco AT, Godinho SA, Yu S, Lemos B (2015) Concerted copy number variation balances ribosomal DNA dosage in human and mouse genomes. Proc Natl Acad Sci 112:2485–2490. https://doi.org/10.1073/pnas.1416878112
Grummt I (2013) The nucleolus—guardian of cellular homeostasis and genome integrity. Chromosoma 122:487–497
Heliot L, Mongelard F, Klein C, O'Donohue MF, Chassery JM, Robert-Nicoud M, Usson Y (2000) Nonrandom distribution of metaphase AgNOR staining patterns on human acrocentric chromosomes. J Histochem Cytochem 48:13–20
Hernandez-Verdun D (2011) Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2:189–194. https://doi.org/10.4161/nucl.2.3.16246
Howell WM, Black DA (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36:1014–1015
Kobayashi T (2008) A new role of the rDNA and nucleolus in the nucleus—rDNA instability maintains genome integrity. Bioessays 30:267–272
Kobayashi T, Sasaki M (2017) Ribosomal DNA stability is supported by many 'buffer genes'-introduction to the yeast rDNA Stability Database. FEMS Yeast Res 17. https://doi.org/10.1093/femsyr/fox001
König K, Linden WA, Canstein M, Baisch H, Canstein L (1975) DNA-synthesis in synchronized L-cells after irradiation during the G1-phase of the cell cycle. Radiat Environ Biophys 12:23–30
Kraemer PM, Petersen DF, Van Dilla MA (1972) DNA constancy in heteroploidy and the stem line theory of tumors. Science 174(4010):714–717
Kurihara Y, Suh DS, Suzuki H, Moriwaki K (1994) Chromosomal locations of Ag-NORs and clusters of ribosomal DNA in laboratory strains of mice. Mamm Genome 5:225–228
Lam YW, Trinkle-Mulcahy L (2015) New insights into nucleolar structure and function. F1000Prime Rep 7:48. https://doi.org/10.12703/P7-48
Leung AK, Gerlich D, Miller G, Lyon C, Lam YW, Lleres D, Daigle N, Zomerdijk J, Ellenberg J, Lamond AI (2004) Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J Cell Biol 166:787–800
McStay B (2016) Nucleolar organizer regions: genomic ‘dark matter’ requiring illumination. Genes Dev 30:1598–1610. https://doi.org/10.1101/gad.283838.116
Mullineux S-T, Lafontaine DLJ (2012) Mapping the cleavage sites on mammalian pre-rRNAs: where do we stand? Biochimie 94:1521–1532. https://doi.org/10.1016/j.biochi.2012.02.001
Nielsén K, Marcus M, Gropp A (1979) Localization of NORs in chromosomes of mouse cell lines by a combined 33258-Hoechst and Ag-staining technique. Hereditas 90:31–37
Németh A, Längst G (2011) Genome organization in and around the nucleolus. Trends Genet 27:149–156. https://doi.org/10.1016/j.tig.2011.01.002
Nishibuchi G, Déjardin J (2017) The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals. Chromosom Res 25:77–87. https://doi.org/10.1007/s10577-016-9547-3
Ostromyshenskii DI, Chernyaeva EN, Kuznetsova IS, Podgornaya OI (2018) Mouse chromocenters DNA content: sequencing and in silico analysis. BMC Genomics 19:151. https://doi.org/10.1186/s12864-018-4534-z
Potapova T, Gorbsky GJ (2017) The consequences of chromosome segregation errors in mitosis and meiosis. Biology (Basel) 6(1):E12. https://doi.org/10.3390/biology6010012
Roussel P, André C, Comai L, Hernandez-Verdun D (1996) The rDNA transcription machinery is assembled during mitosis in active NORs and absent in inactive NORs. J Cell Biol 133:235–246
Seither P, Zatsepina O, Hoffmann M, Grummt I (1997) Constitutive and strong association of PAF53 with RNA polymerase I. Chromosoma 106:216–225
Sirri V, Roussel P, Hernandez-Verdun D (1999) The mitotically phosphorylated form of the transcription termination factor TTF-1 is associated with the repressed rDNA transcription machinery. J Cell Sci 112:3259–3268
Sirri V, Roussel P, Hernandez-Verdun D (2000) The AgNOR proteins: qualitative and quantitative changes during the cell cycle. Micron 31:121–126
Smirnov E, Kalmárová M, Koberna K, Zemanová Z, Malínský J, Masata M, Cvacková Z, Michalová K, Raska I (2006) NORs and their transcription competence during the cell cycle. Folia Biol (Praha) 52:59–70
Sobecki D, Mrouj K, Camasses A et al (2016) The cell proliferation antigen Ki-67 organises heterochromatin. Elife 5:e13722. https://doi.org/10.7554/eLife.13722
Sorokina EA, Novikova II, Grinchuk TM, Sal'nikov KV (1988) Karyotype analysis of clone L929 murine fibroblasts by using differential chromosome staining. Tsitologiia 30:197–204
Strobel RJ, Pathak S, Hsu TC (1981) NOR lateral asymmetry and its effect on satellite association in BrdU-labeled human lymphocyte cultures. Hum Genet 59:259–262
Suzuki H, Kurihara Y, Kanehisa T, Moriwaki K (1990) Variation in the distribution of silver-staining nucleolar organizer regions on the chromosomes of the wild mouse, Mus musculus. Mol Biol Evol 7:271–282
Tiku V, Antebi A (2018) Nucleolar function in lifespan regulation. Trends Cell Biol 28:662–672. https://doi.org/10.1016/j.tcb.2018.03.007
Tsekrekou M, Stratigi K, Chatzinikolaou G (2017) The nucleolus: in genome maintenance and repair. Int J Mol Sci 18:E1411. https://doi.org/10.3390/ijms18071411
Valdez BC, Henning D, So RB, Dixon J, Dixon MJ (2004) The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci 101:10709–10714
Worrall JT, Tamura N, Mazzagatti A, Shaikh N, van Lingen T, Bakker B, Spierings DCJ, Vladimirou E, Foijer F, McClelland SE (2018) Non-random mis-segregation of human chromosomes. Cell Rep 23(11):3366–3380. https://doi.org/10.1016/j.celrep.2018.05.047
Zatsepina OV, Zharskaya OO, Prusov AN (2008) Isolation of the constitutive heterochromatin from mouse liver nuclei. In: R. Hancock (Ed) The nucleus: nuclei and subnuclear components. Humana Press, NY. Chapter 12, p. 167–177
Zatsepina OV, Voit R, Grummt I, Spring H, Semenov MV, Trendelenburg MF (1993) The RNA polymerase I-specific transcription initiation factor UBF is associated with transcriptionally active and inactive ribosomal genes. Chromosoma 102:599–611
Acknowledgements
The author is grateful to Prof. Ingrid Grummt (German Cancer Research Center, Heidelberg, Germany) for a kind provision of the rDNA plasmids, and to Drs. Maria Chaplina, Iana Riabukha, and Anastasiya Moraleva (Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry RAS, Moscow, Russia) for a valuable assistance in some experiments (MC) and statistical evaluation of the selected data (IR, AM).
Funding
The study was supported by the Russian Foundation for Basic Research (grant 16-04-01199).
Author information
Authors and Affiliations
Contributions
The author designed the study, participated in the majority of experiments, performed a statistical analysis, wrote the paper, and prepared the illustrations.
Corresponding author
Additional information
Jennifer Gerton and Lev Porokhovnik
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 141 kb)
Rights and permissions
About this article
Cite this article
Zatsepina, O.V. Cytogenetic instability of chromosomal nucleolar organizer regions (NORs) in cloned mouse L929 fibroblasts. Chromosome Res 27, 95–108 (2019). https://doi.org/10.1007/s10577-018-9598-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-018-9598-8