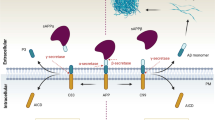
Abstract
STEP (STriatal-Enriched Protein Tyrosine Phosphatase) is a brain-specific phosphatase that plays an important role in controlling signaling molecules involved in neuronal activity and synaptic development. The striatum is the main location of the STEP enzyme. An imbalance in STEP61 activity is a risk factor for Alzheimer’s disease (AD). It can contribute to the development of numerous neuropsychiatric diseases, including Parkinson’s disease (PD), schizophrenia, fragile X syndrome (FXS), Huntington’s disease (HD), alcoholism, cerebral ischemia, and stress-related diseases. The molecular structure, chemistry, and molecular mechanisms associated with STEP61’s two major substrates, Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPAr) and N-methyl-d-aspartate receptors (NMDARs), are crucial in understanding the relationship between STEP61 and associated illnesses. STEP’s interactions with its substrate proteins can alter the pathways of long-term potentiation and long-term depression. Therefore, understanding the role of STEP61 in neurological illnesses, particularly Alzheimer’s disease-associated dementia, can provide valuable insights for possible therapeutic interventions. This review provides valuable insights into the molecular structure, chemistry, and molecular mechanisms associated with STEP61. This brain-specific phosphatase controls signaling molecules involved in neuronal activity and synaptic development. This review can aid researchers in gaining deep insights into the complex functions of STEP61.




Similar content being viewed by others
Data Availability
The data used in this review article are primarily derived from online available sources, including academic journals, books and reputable online databases. The specific datasets and studies referenced in this review are appropriately cited within the text. Supplementary data were not utilized in the analysis. Further details regarding the sources of data can be found in the Material and Methods section of this article.
Abbreviations
- AD:
-
Alzheimer’s disease
- AMPAr:
-
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor
- APP:
-
Amyloid precursor protein
- Aβ:
-
Amyloid beta
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- Cyclic AMP:
-
Cyclic adenosine mono phosphate
- DARPP-32:
-
Dopamine- and camp-regulated neuronal phosphoprotein
- DR1:
-
Dopamine receptor1
- EGF:
-
Epidermal growth factor
- ERK1/2:
-
Extracellular signal‑regulated protein kinase
- FMR1:
-
Fragile X mental retardation protein
- FSH:
-
Follicle-stimulating hormone
- FXS:
-
Fragile X syndrome
- Fyn:
-
Proto-oncogene tyrosine-protein kinase
- Glua1/2/3/4:
-
GRIA2
- Glun2b:
-
GRIN2B
- HD:
-
Huntington’s disease
- IL-1B:
-
Interleukin 1 beta
- KIM:
-
Kinase interacting motif domain
- KISS:
-
Kinase specificity sequence domain
- LTD:
-
Long-term depression
- LTP:
-
Long-term potentiation
- MAPK1/3/11/12/13/14:
-
Mitogen-activated protein kinase
- Mhtt:
-
Mutant huntington
- NCBI:
-
National Center for Biotechnology Information
- NMDAr:
-
N-Methyl-d-aspartate receptor
- NRG1:
-
Neuregulin 1
- P38:
-
A group of mitogen-activated protein kinases
- PD:
-
Parkinson’s disease
- PEST:
-
Proline, glutamic acid, serine, threonine
- PKA:
-
Protein kinase A
- PP1:
-
Protein phosphatase 1
- PPIs:
-
Protein–protein interactions
- PPP1R1B:
-
Protein phosphatase 1 regulatory subunit 1B
- PSD-95:
-
Post-synaptic density protein 95
- PTK2B:
-
Protein tyrosine kinase 2beta
- PTP:
-
Phosphatase domain
- PTP1B:
-
Protein tyrosine phosphatase 1B
- PTPN5:
-
Tyrosine-protein phosphatase non-receptor type 5
- Pyk2:
-
Proline-rich tyrosine kinase 2
- Q/R Editing:
-
Replacement of amino acid glutamine by arginine
- SHP2:
-
Src homology region 2 containing protein tyrosine phosphatase 2
- STEP61:
-
STriatal-Enriched protein tyrosine Phosphatase
- STRING (string-db.org):
-
String interactome database
- SZ:
-
Schizophrenia
- Tnfα:
-
Tumor necrosis factor Α
- UPS:
-
Ubiquitin–proteasome system
- WPD:
-
Tryptophan, proline, and aspartic acid loop
- α7nachrs:
-
Alpha7 nicotinic acetylcholine receptor
References
Ashrafian H, Zadeh EH, Khan RH (2021) Review on Alzheimer’s disease: inhibition of amyloid beta and tau tangle formation. Int J Biol Macromol 167:382–394. https://doi.org/10.1016/J.IJBIOMAC.2020.11.192
Barr AJ, Ugochukwu E, Lee WH et al (2009) Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 136:352–363. https://doi.org/10.1016/J.CELL.2008.11.038
Barria A, Malinow R (2002) Subunit-specific NMDA receptor trafficking to synapses. Neuron 35:345–353. https://doi.org/10.1016/s0896-6273(02)00776-6
Blanco-Aparicio C, Torres J, Pulido R (1999) A novel regulatory mechanism of MAP kinases activation and nuclear translocation mediated by PKA and the PTP-SL tyrosine phosphatase. J Cell Biol 147:1129–1135. https://doi.org/10.1083/jcb.147.6.1129
Bliss TVP, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. https://doi.org/10.1038/361031a0
Boulanger LM, Lombroso PJ, Raghunathan A et al (1995) Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci 15:1532–1544. https://doi.org/10.1523/jneurosci.15-02-01532.1995
Braithwaite SP, Adkisson M, Leung J et al (2006) Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP). Eur J Neurosci 23:2847–2856. https://doi.org/10.1111/j.1460-9568.2006.04837.x
Braithwaite SP, Xu J, Leung J et al (2008) Expression and function of striatal enriched protein tyrosine phosphatase is profoundly altered in cerebral ischemia. Eur J Neurosci 27:2444–2452. https://doi.org/10.1111/j.1460-9568.2008.06209.x
Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40:361–379. https://doi.org/10.1016/s0896-6273(03)00640-8
Bronzuoli MR, Iacomino A, Steardo L, Scuderi C (2022) Targeting neuroinflammation in Alzheimer’s disease. J Inflamm Res 9:199–208. https://doi.org/10.2147/JIR.S86958
Bult A, Zhao F, Dirkx R et al (1996) STEP61: a member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci 16:7821–7831. https://doi.org/10.1523/jneurosci.16-24-07821.1996
Bult A, Zhao F, Dirkx R et al (1997) STEP: a family of brain-enriched PTPs. Alternative splicing produces transmembrane, cytosolic and truncated isoforms. Eur J Cell Biol 72:337–344
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimer Dementia 12:719–732
Carty NC (2012) The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry 2:137
Carvajal FJ, Cerpa W, Lazzarino G, Amorini AM (2021) Regulation of phosphorylated state of NMDA receptor by STEP61 phosphatase after mild-traumatic brain injury: role of oxidative stress. Antioxidants 10:1575. https://doi.org/10.3390/ANTIOX10101575
Caselli RJ, Beach TG, Knopman DS, Graff-Radford NR (2017) Alzheimer disease: scientific breakthroughs and translational challenges. Mayo Clin Proc 92:978–994. https://doi.org/10.1016/j.mayocp.2017.02.011
Chater TE, Goda Y (2014) The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci 8:401. https://doi.org/10.3389/fncel.2014.00401
Chatterjee M, Kwon J, Benedict J et al (2021) STEP inhibition prevents Aβ-mediated damage in dendritic complexity and spine density in Alzheimer’s disease. Exp Brain Res 239:881–890. https://doi.org/10.1007/s00221-020-06028-x
Chizhova N, Khomenko T, Volcho K, Amstislavskaya T (2022) Antipsychotic activity of a new PTPN5 blocker (TC-2051) in mice with the Disc1-L100P mutation. scholar.archive.org
Dewang PM, Hsu N-M, Peng S-Z, Li W-R (2005) Protein tyrosine phosphatases and their inhibitors. Curr Med Chem 12:1–22
Drummond E, Kavanagh T, Pires G et al (2022) The amyloid plaque proteome in early onset Alzheimer’s disease and Down syndrome. Acta Neuropathol Commun. https://doi.org/10.1186/S40478-022-01356-1
Durakoglugil MS, Wasser CR, Wong CH et al (2021) Reelin regulates neuronal excitability through striatal-enriched protein tyrosine phosphatase (STEP61) and calcium permeable AMPARs in an NMDAR-dependent manner. J Neurosci 41:7340–7349. https://doi.org/10.1523/JNEUROSCI.0388-21.2021
Eswaran J, von Kries JP, Marsden B et al (2006) Crystal structures and inhibitor identification for PTPN5, PTPRR and PTPN7: a family of human MAPK-specific protein tyrosine phosphatases. Biochem J 395:483–491. https://doi.org/10.1042/BJ20051931
Fitzpatrick CJ, Lombroso PJ (2011) The role of striatal-enriched protein tyrosine phosphatase (STEP) in cognition. Front Neuroanat. https://doi.org/10.3389/FNANA.2011.00047/FULL
Folch J, Petrov D, Ettcheto M et al (2016) Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast. https://doi.org/10.1155/2016/8501693
Francis DM, Koveal D, Tortajada A et al (2014) Interaction of kinase-interaction-motif protein tyrosine phosphatases with the mitogen-activated protein kinase ERK2. PLoS ONE 9:e91934. https://doi.org/10.1371/JOURNAL.PONE.0091934
Frontzkowski L, Ewers M, Brendel M et al (2022) Earlier Alzheimer’s disease onset is associated with tau pathology in brain hub regions and facilitated tau spreading. Nat Commun. https://doi.org/10.1038/S41467-022-32592-7
Furukawa H, Singh SK, Mancusso R, Gouaux E (2005) Subunit arrangement and function in NMDA receptors. Nature 438:185–192. https://doi.org/10.1038/nature04089
Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG (2009) Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci 29:6479–6489. https://doi.org/10.1523/jneurosci.3753-08.2009
Gee CE, Benquet P, Raineteau O et al (2006) NMDA receptors and the differential ischemic vulnerability of hippocampal neurons. Wiley Online Library 23:2595–2603. https://doi.org/10.1111/j.1460-9568.2006.04786.x
Goebel-Goody SM (2012) Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev 64:65–87
Gouras GK, Olsson TT, Hansson O (2015) β-amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 12:3–11. https://doi.org/10.1007/S13311-014-0313-Y
Guntupalli S, Widagdo J, Anggono V (2016) Amyloid-β-induced dysregulation of AMPA receptor trafficking. Neural Plast. https://doi.org/10.1155/2016/3204519
Hayashi T, Huganir RL (2004) Tyrosine phosphorylation and regulation of the AMPA receptor by Src family tyrosine kinases. J Neurosci 24:6152–6160. https://doi.org/10.1523/jneurosci.0799-04.2004
Hee JC, Yan HH, Lau LF, Huganir RL (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 24:10248–10259. https://doi.org/10.1523/jneurosci.0546-04.2004
Heneberg P (2009) Use of protein tyrosine phosphatase inhibitors as promising targeted therapeutic drugs. Curr Med Chem 16:706–733
Henley JM, Wilkinson KA (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci 17:337–350. https://doi.org/10.1038/nrn.2016.37
Herguedas B, García-Nafría J, Cais O et al (2016) Structure and organization of heteromeric AMPA-type glutamate receptors. Science (1979). https://doi.org/10.1126/science.aad3873
Hsieh H, Boehm J, Sato C et al (2006) AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 52:831–843. https://doi.org/10.1016/J.NEURON.2006.10.035
Iacobucci GJ, Popescu GK (2017) NMDA receptors: linking physiological output to biophysical operation. Nat Rev Neurosci 18:236–249. https://doi.org/10.1038/nrn.2017.24
Isaac JTR, Ashby M, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871. https://doi.org/10.1016/J.NEURON.2007.06.001
Jacobi E, von Engelhardt J (2017) Diversity in AMPA receptor complexes in the brain. Curr Opin Neurobiol 45:32–38. https://doi.org/10.1016/j.conb.2017.03.001
Jang SS, Royston SE, Xu J et al (2015) Regulation of STEP61 and tyrosine-phosphorylation of NMDA and AMPA receptors during homeostatic synaptic plasticity. Mol Brain 8:55. https://doi.org/10.1186/s13041-015-0148-4
Jang SS, Royston SE, Lee G et al (2016) Seizure-induced regulations of amyloid-β, STEP61, and STEP61 substrates involved in hippocampal synaptic plasticity. Neural Plast. https://doi.org/10.1155/2016/2123748
Katsel P, Haroutunian V (2019) State of the art Is Alzheimer disease a failure of mobilizing immune defense? Lessons from cognitively fit oldest-old. Dialogues Clin Neurosci 21:7–19
Kaufman SK, Del Tredici K, Thomas TL et al (2018) Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol 136:57–67. https://doi.org/10.1007/S00401-018-1855-6
Kerchner GA, Nicoll RA (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9:813–825. https://doi.org/10.1038/nrn2501
Kulikova EA, Fursenko, Bazhenova EY, Kulikov A D (2021) Decrease in the activity of striatal-enriched protein-tyrosine-phosphatase (STEP) in the brain of Danio rerio treated with p-chlorophenylalanine and Pargyline. Mol Biol 55:604–609. https://doi.org/10.1134/S0026893321020254/FIGURES/4
Kurup P, Zhang Y, Xu J et al (2010) Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci 30:5948–5957. https://doi.org/10.1523/JNEUROSCI.0157-10.2010
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25:59–70. https://doi.org/10.1111/ene.13439
Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426. https://doi.org/10.1038/nrn2153
Lavezzari G, McCallum J, Dewey CM, Roche KW (2004) Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci 24:6383–6391. https://doi.org/10.1523/jneurosci.1890-04.2004
Leng F, Hinz R, Gentleman S et al (2022) Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Mol Psychiatry 28:1303–1311. https://doi.org/10.1038/s41380-022-01878-z
Lind GE, Mou TC, Tamborini L et al (2017) Structural basis of subunit selectivity for competitive NMDA receptor antagonists with preference for GluN2A over GluN2B subunits. Proc Natl Acad Sci USA 114:E6942–E6951. https://doi.org/10.1073/pnas.1707752114
Liu SJ, Zukin RS (2007) Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134. https://doi.org/10.1016/J.TINS.2007.01.006
Lombroso PJ, Murdoch G, Lerner M (1991) Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. PNAS 88:7242–7246. https://doi.org/10.1073/pnas.88.16.7242
Lu W, Shi Y, Jackson AC et al (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62:254–268. https://doi.org/10.1016/j.neuron.2009.02.027
Lüscher C, Nicoll RA, Malenka RC, Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci 3:545–550. https://doi.org/10.1038/75714
Lussier MP, Herring BE, Nasu-Nishimura Y et al (2012) Ubiquitin ligase RNF167 regulates AMPA receptor-mediated synaptic transmission. Proc Natl Acad Sci USA 109:19426–19431. https://doi.org/10.1073/pnas.1217477109
Lussier MP, Sanz-Clemente A, Roche KW (2015) Dynamic regulation of N-Methyl-d-aspartate (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by posttranslational modifications. J Biol Chem 290:28596–28603. https://doi.org/10.1074/jbc.r115.652750
Mahaman YAR, Huang F, Embaye KS et al (2021) The implication of STEP in synaptic plasticity and cognitive impairments in Alzheimer’s disease and other neurological disorders. Front Cell Dev Biol 9:680118. https://doi.org/10.3389/fcell.2021.680118
Masters CL, Bateman R, Blennow K et al (2015) Alzheimer’s disease. Nat Rev Dis Primers 1:15056. https://doi.org/10.1038/nrdp.2015.56
Morisot N, Ron D (2017) Alcohol-dependent molecular adaptations of the NMDA receptor system. Genes Brain Behav 16:139–148. https://doi.org/10.1111/GBB.12363
Moskaliuk VS, Kozhemyakina R, Bazovkina D et al (2022) On an association between fear-induced aggression and striatal-enriched protein tyrosine phosphatase (STEP) in the brain of Norway rats. Biomed Pharmacother 147:112667. https://doi.org/10.1016/J.BIOPHA.2022.112667
Muñoz JJ, Tárrega C, Blanco-Aparicio C, Pulido R (2003) Differential interaction of the tyrosine phosphatases PTP-SL, STEP and HePTP with the mitogen-activated protein kinases ERK1/2 and p38alpha is determined by a kinase specificity sequence and influenced by reducing agents. Biochem J 372:193–201. https://doi.org/10.1042/bj20021941
Murphy MP, Levine H (2010) Alzheimer’s disease and the β-amyloid peptide. J Alzheimers Dis 19:311. https://doi.org/10.3233/JAD-2010-1221
Murray ME, Graff-Radford NR, Ross OA et al (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796. https://doi.org/10.1016/s1474-4422(11)70156-9
Nakazawa T, Komai S, Tezuka T et al (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem 276:693–699. https://doi.org/10.1074/jbc.m008085200
Nazem A, Sankowski R, Bacher M, Al-Abed Y (2015) Rodent models of neuroinflammation for Alzheimer’s disease. J Neuroinflammation. https://doi.org/10.1186/S12974-015-0291-Y
Nguyen TH, Liu J, Lombroso PJ (2002) Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem 277:24274–24279. https://doi.org/10.1074/jbc.m111683200
Nicoll RA, Tomita S, Bredt DS (2006) Auxiliary subunits assist AMPA-type glutamate receptors. Science 311:1253–1256. https://doi.org/10.1126/SCIENCE.1123339
Ovsepian SV, O’Leary VB (2016) Neuronal activity and amyloid plaque pathology: an update. J Alzheimer’s Dis 49(1):13–19
Palop JJ, Mucke L (2010) Amyloid-Β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 13:812–818. https://doi.org/10.1038/NN.2583
Pan L, Li T, Wang R et al (2022) Roles of phosphorylation of N-methyl-d-aspartate receptor in chronic pain. Cell Mol Neurobiol. https://doi.org/10.1007/S10571-022-01188-6/TABLES/1
Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14:383–400. https://doi.org/10.1038/nrn3504
Paul S, Connor JA (2010) NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem 114:1107–1118. https://doi.org/10.1111/J.1471-4159.2010.06835.X
Paul S, Nairn AC, Wang P, Lombroso PJ (2003) NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci 6:34–42. https://doi.org/10.1038/nn989
Paul S, Olausson P, Venkitaramani D et al (2007) The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry 61:1049–1061. https://doi.org/10.1016/j.biopsych.2006.08.005
Pelkey KA, Askalan R, Paul S et al (2002) Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron 34:127–138. https://doi.org/10.1016/s0896-6273(02)00633-5
Perl DP (2010) Neuropathology of Alzheimer’s disease. Mt Sinai J Med 77:32–42. https://doi.org/10.1002/msj.20157
Pimplikar SW (2014) Neuroinflammation in Alzheimer’s disease: From pathogenesis to a therapeutic target. J Clin Immunol. https://doi.org/10.1007/S10875-014-0032-5
Poddar R, Deb I, Mukherjee S, Paul S (2010) NR2B-NMDA receptor mediated modulation of the tyrosine phosphatase STEP regulates glutamate induced neuronal cell death. J Neurochem 115:1350–1362. https://doi.org/10.1111/J.1471-4159.2010.07035.X
Roche KW, Standley S, McCallum J et al (2001) Molecular determinants of NMDA receptor internalization. Nat Neurosci 4:794–802. https://doi.org/10.1038/90498
Rodriguez-Vieitez E, Montal V, Sepulcre J et al (2021) Association of cortical microstructure with amyloid-β and tau: impact on cognitive decline, neurodegeneration, and clinical progression in older adults. Mol Psychiatry 26:7813–7822. https://doi.org/10.1038/S41380-021-01290-Z
Roth RH, Zhang Y, Huganir RL (2017) Dynamic imaging of AMPA receptor trafficking in vitro and in vivo. Curr Opin Neurobiol 45:51–58. https://doi.org/10.1016/j.conb.2017.03.008
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–344. https://doi.org/10.1128/MMBR.68.2.320-344.2004
Rush T, Buisson A (2014) Reciprocal disruption of neuronal signaling and Aβ production mediated by extrasynaptic NMDA receptors: a downward spiral. Cell Tissue Res 356:279–286. https://doi.org/10.1007/s00441-013-1789-1
Sanz-Clemente A, Matta JA, Isaac JTR, Roche KW (2010) Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67:984–996. https://doi.org/10.1016/j.neuron.2010.08.011
Sanz-Clemente A, Nicoll RA, Roche KW (2013) Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist 19:62–75. https://doi.org/10.1177/1073858411435129
Scheltens P, De Strooper B, Kivipelto M et al (2021) Alzheimer’s disease. The Lancet 397:1577–1590
Shi SH, Hayashi Y, Esteban JA, Malinow R (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105:331–343. https://doi.org/10.1016/s0092-8674(01)00321-x
Snyder EM, Nong Y, Almeida CG et al (2005) Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci 8:1051–1058. https://doi.org/10.1038/nn1503
Sobolevsky AI, Rosconi MP, Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462:745–756. https://doi.org/10.1038/nature08624
Szedlacsek HS, Bajusz D, Badea RA et al (2022) Designed peptide inhibitors of STEP phosphatase-GluA2 AMPA receptor interaction enhance the cognitive performance in rats. J Med Chem 65:217–233. https://doi.org/10.1021/ACS.JMEDCHEM.1C01303/SUPPL_FILE/JM1C01303_SI_002.CSV
Szklarczyk D, Gable AL, Lyon D et al (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607–D613. https://doi.org/10.1093/NAR/GKY1131
Szklarczyk D, Gable AL, Nastou KC et al (2021) The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49:D605–D612. https://doi.org/10.1093/NAR/GKAA1074
Takahashi RH, Nagao T, Gouras GK (2017) Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol Int 67:185–193. https://doi.org/10.1111/PIN.12520
Tautermann CS, Binder F, Büttner FH et al (2019) Allosteric activation of striatal-enriched protein tyrosine phosphatase (STEP, PTPN5) by a fragment-like molecule. J Med Chem 62:306–316. https://doi.org/10.1021/ACS.JMEDCHEM.8B00857
Taylor D (2022) The role of the phosphatase STEP and kinase Fyn in neurophysiology and pathology
Taylor D, Kneynsberg A, van Roijen M, Götz J (2023) Tyrosine phosphatase STEP61 in human dementia and in animal models with amyloid and tau pathology. Mol Brain. https://doi.org/10.1186/S13041-023-00994-3
Thal DR, Attems J, Ewers M (2014) Spreading of Amyloid, Tau, and microvascular pathology in Alzheimer’s disease: findings from neuropathological and neuroimaging studies. J Alzheimer’s Dis 42:S421–S429. https://doi.org/10.3233/JAD-141461
Walters JM, Kim EC, Zhang J et al (2022) Pharmacological inhibition of STriatal-Enriched protein tyrosine Phosphatase by TC-2153 reduces hippocampal excitability and seizure propensity. Epilepsia. https://doi.org/10.1111/EPI.17192
Wang H, Bu S, Tang J et al (2021) PTPN5 promotes follicle-stimulating hormone secretion through regulating intracellular calcium homeostasis. FASEB J 35:e21756. https://doi.org/10.1096/FJ.202002752RR
Wei H, Zhang H, Wang X et al (2020) Direct activation of protein phosphatase 2A (PP2A) by tricyclic sulfonamides ameliorates Alzheimer’s disease pathogenesis in cell and animal models. Neurotherapeutics 17:1087–1103. https://doi.org/10.1007/S13311-020-00841-6/FIGURES/6
Widagdo J, Guntupalli S, Jang SE, Anggono V (2017) Regulation of AMPA receptor trafficking by protein ubiquitination. Front Mol Neurosci 10:347
Witten MR, Wissler L, Snow M et al (2017) X-ray characterization and structure-based optimization of striatal-enriched protein tyrosine phosphatase inhibitors. J Med Chem 60:9299–9319. https://doi.org/10.1021/ACS.JMEDCHEM.7B01292
Won S, Incontro S, Li Y et al (2019) The STEP61 interactome reveals subunit-specific AMPA receptor binding and synaptic regulation. Proc Natl Acad Sci USA 116:8028–8037. https://doi.org/10.1073/pnas.1900878116
Won S, Roche KW, Forsythe I et al (2021) Regulation of glutamate receptors by striatal-enriched tyrosine phosphatase 61 (STEP61). Wiley Online Library 599:443–451. https://doi.org/10.1113/JP278703
Xu J, Kurup P, Zhang Y et al (2009) Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci 29:9330–9343. https://doi.org/10.1523/JNEUROSCI.2212-09.2009
Xu J, Kurup P, Bartos JA et al (2012) Striatal-enriched protein-tyrosine phosphatase (STEP) regulates Pyk2 kinase activity. J Biol Chem 287:20942–20956. https://doi.org/10.1074/JBC.M112.368654/ATTACHMENT/208D8A37-B94A-4611-8F56-5182B184BFF0/MMC1.PDF
Xu J, Chatterjee M, Baguley TD et al (2014) Inhibitor of the tyrosine phosphatase step reverses cognitive deficits in a mouse model of alzheimer’s disease. PLoS Biol. https://doi.org/10.1371/JOURNAL.PBIO.1001923
Xu J, Kurup P, Nairn AC, Lombroso PJ (2018) Synaptic NMDA receptor activation induces ubiquitination and degradation of STEP61. Mol Neurobiol 55:3096–3111. https://doi.org/10.1007/s12035-017-0555-x
Zhang Z-Y (2001) Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol 42(1):209–234
Zhang F, Jiang L (2015) Neuroinflammation in Alzheimer’s disease. Neuropsychiatr Dis Treat 11:243–256
Zhang L, Wang H (2020) FTY720 in CNS injuries: Molecular mechanisms and therapeutic potential. Brain Res Bull 164:75–82. https://doi.org/10.1016/J.BRAINRESBULL.2020.08.013
Zhang Y, Venkitaramani D, Gladding CM et al (2008) The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci 28:10561–10566. https://doi.org/10.1523/jneurosci.2666-08.2008
Zhang Y, Kurup P, Xu J et al (2010) Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive andcellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA 107:19014–19019. https://doi.org/10.1073/pnas.1013543107
Zhang Y, Kurup P, Xu J et al (2011) Reduced levels of the tyrosine phosphatase STEP block beta amyloid-mediated GluA1/GluA2 receptor internalization. J Neurochem 119:664–672. https://doi.org/10.1111/j.1471-4159.2011.07450.x
Zhang L, Xie JW, Yang J, Cao YP (2013) Tyrosine phosphatase STEP61 negatively regulates amyloid β-mediated ERK/CREB signaling pathways via α7 nicotinic acetylcholine receptors. J Neurosci Res 91:1581–1590. https://doi.org/10.1002/jnr.23263
Zhang L, Yang J, Liu ZH (2018) Step61 negatively regulates amyloid beta-mediated erk signaling pathway in alzheimer’s disease cell model. Chin J Tissue Eng Res 22:4507–4512. https://doi.org/10.3969/J.ISSN.2095-4344.0796
Zhao Y, Chen S, Swensen AC et al (2019) Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 364:355–362. https://doi.org/10.1126/SCIENCE.AAW8250
Zhao X, Xiong L, She L et al (2022) The role and therapeutic implication of protein tyrosine phosphatases in Alzheimer’s disease. Biomed Pharmacother. https://doi.org/10.1016/J.BIOPHA.2022.113188
Zou J, Lei T, Guo P et al (2019) Mechanisms shaping the role of ERK1/2 in cellular senescence (Review). Mol Med Rep 19:759–770. https://doi.org/10.3892/MMR.2018.9712
Acknowledgements
Not applicable.
Funding
This work was supported by an Indo-Hungary Grant received by Prof. Sadhana Sathaye from the Department of Pharmaceutical Sciences and Technology.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s design, while PVB and RDD performed data collection and literature review. PVB prepared the first draft of the manuscript, and all authors provided feedback on previous versions. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Research Involving of Human and Animal Participants
This article contains no studies with human and animal subjects performed by any author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bagwe, P.V., Deshpande, R.D., Juhasz, G. et al. Uncovering the Significance of STEP61 in Alzheimer’s Disease: Structure, Substrates, and Interactome. Cell Mol Neurobiol 43, 3099–3113 (2023). https://doi.org/10.1007/s10571-023-01364-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-023-01364-2