Abstract
Tripartite motif (TRIM) protein superfamily is a group of E3 ubiquitin ligases characterized by the conserved RING domain, the B-box domain, and the coiled-coil domain (RBCC). It is widely involved in various physiological and pathological processes, such as intracellular signal transduction, cell cycle regulation, oncogenesis, and innate immune response. Central nervous system (CNS) diseases are composed of encephalopathy and spinal cord diseases, which have a high disability and mortality rate. Patients are often unable to take care of themselves and their life quality can be seriously declined. Initially, the function research of TRIM proteins mainly focused on cancer. However, in recent years, accumulating attention is paid to the roles they play in CNS diseases. In this review, we integrate the reported roles of TRIM proteins in the pathological process of CNS diseases and related signaling pathways, hoping to provide theoretical bases for further research in treating CNS diseases targeting TRIM proteins.
Graphical Abstract
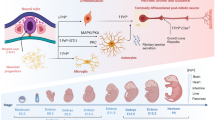
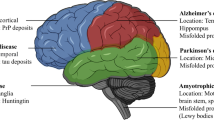
TRIM proteins participated in CNS diseases. TRIM protein family is characterized by a highly conserved RBCC domain, referring to the RING domain, the B-box domain, and the coiled-coil domain. Recent research has discovered the relations between TRIM proteins and various CNS diseases, especially Alzheimer’s disease, Parkinson’s disease, and ischemic stroke.





Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Abate G, Frisoni GB, Bourdon JC, Piccirella S, Memo M, Uberti D (2020) The pleiotropic role of p53 in functional/dysfunctional neurons: focus on pathogenesis and diagnosis of Alzheimer’s disease. Alzheimers Res Ther 12(1):160. https://doi.org/10.1186/s13195-020-00732-0
Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270(46):27489–27494. https://doi.org/10.1074/jbc.270.46.27489
Bai X, Zhang YL, Liu LN (2020) Inhibition of TRIM8 restrains ischemia-reperfusion-mediated cerebral injury by regulation of NF-kappaB activation associated inflammation and apoptosis. Exp Cell Res 388(2):111818. https://doi.org/10.1016/j.yexcr.2020.111818
Bai X, Fu RJ, Zhang S, Yue SJ, Chen YY, Xu DQ, Tang YP (2021) Potential medicinal value of celastrol and its synthesized analogues for central nervous system diseases. Biomed Pharmacother 139:111551. https://doi.org/10.1016/j.biopha.2021.111551
Balastik M, Ferraguti F, Pires-da Silva A, Lee TH, Alvarez-Bolado G, Lu KP, Gruss P (2008) Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc Natl Acad Sci U S A 105(33):12016–12021. https://doi.org/10.1073/pnas.0802261105
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2):263–265. https://doi.org/10.1093/bioinformatics/bth457
Basu-Shrivastava M, Kozoriz A, Desagher S, Lassot I (2021) To ubiquitinate or not to ubiquitinate: TRIM17 in cell life and death. Cells 10(5):1235. https://doi.org/10.3390/cells10051235
Bell JL, Malyukova A, Holien JK, Koach J, Parker MW, Kavallaris M, Marshall GM, Cheung BB (2012) TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PLoS ONE 7(5):e37470. https://doi.org/10.1371/journal.pone.0037470
Bernardi R, Pandolfi PP (2007) Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol 8(12):1006–1016. https://doi.org/10.1038/nrm2277
Boillee S, Vande Velde C, Cleveland DW (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52(1):39–59. https://doi.org/10.1016/j.neuron.2006.09.018
Boyer NP, McCormick LE, Menon S, Urbina FL, Gupton SL (2020) A pair of E3 ubiquitin ligases compete to regulate filopodial dynamics and axon guidance. J Cell Biol. https://doi.org/10.1083/jcb.201902088
Braak HEB (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Buizza L, Cenini G, Lanni C, Ferrari-Toninelli G, Prandelli C, Govoni S, Buoso E, Racchi M, Barcikowska M, Styczynska M, Szybinska A, Butterfield DA, Memo M, Uberti D (2012) Conformational altered p53 as an early marker of oxidative stress in Alzheimer’s disease. PLoS ONE 7(1):e29789. https://doi.org/10.1371/journal.pone.0029789
Cammas F, Mark M, Dolle P, Dierich A, Chambon P, Losson R (2000) Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development 127(13):2955–2963. https://doi.org/10.1242/dev.127.13.2955
Cao L, Chen J, Li M, Qin YY, Sun M, Sheng R, Han F, Wang G, Qin ZH (2015) Endogenous level of TIGAR in brain is associated with vulnerability of neurons to ischemic injury. Neurosci Bull 31(5):527–540. https://doi.org/10.1007/s12264-015-1538-4
Caraveo G, Auluck PK, Whitesell L, Chung CY, Baru V, Mosharov EV, Yan X, Ben-Johny M, Soste M, Picotti P, Kim H, Caldwell KA, Caldwell GA, Sulzer D, Yue DT, Lindquist S (2014) Calcineurin determines toxic versus beneficial responses to alpha-synuclein. Proc Natl Acad Sci U S A 111(34):E3544-3552. https://doi.org/10.1073/pnas.1413201111
Chang R, Xu X, Li MD (2002) Molecular cloning, mapping and characterization of a novel mouse RING finger gene, Mrf1. Gene 291(1–2):241–249. https://doi.org/10.1016/s0378-1119(02)00603-0
Chen L, Brewer MD, Guo L, Wang R, Jiang P, Yang X (2017) Enhanced degradation of misfolded proteins promotes tumorigenesis. Cell Rep 18(13):3143–3154. https://doi.org/10.1016/j.celrep.2017.03.010
Chen L, Zhu G, Johns EM, Yang X (2018) TRIM11 activates the proteasome and promotes overall protein degradation by regulating USP14. Nat Commun 9(1):1223. https://doi.org/10.1038/s41467-018-03499-z
Chen RH, Chen YH, Huang TY (2019) Ubiquitin-mediated regulation of autophagy. J Biomed Sci 26(1):80. https://doi.org/10.1186/s12929-019-0569-y
Chen Z, Wu H, Zhang M (2021) Long non-coding RNA: An underlying bridge linking neuroinflammation and central nervous system diseases. Neurochem Int 148:105101. https://doi.org/10.1016/j.neuint.2021.105101
Cheng CT, Kuo CY, Ann DK (2014) KAPtain in charge of multiple missions: emerging roles of KAP1. World J Biol Chem 5(3):308–320. https://doi.org/10.4331/wjbc.v5.i3.308
Chhor V, Moretti R, Le Charpentier T, Sigaut S, Lebon S, Schwendimann L, Ore MV, Zuiani C, Milan V, Josserand J, Vontell R, Pansiot J, Degos V, Ikonomidou C, Titomanlio L, Hagberg H, Gressens P, Fleiss B (2017) Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav Immun 63:197–209. https://doi.org/10.1016/j.bbi.2016.11.001
Cho B, Choi SY, Cho HM, Kim HJ, Sun W (2013) Physiological and pathological significance of dynamin-related protein 1 (drp1)-dependent mitochondrial fission in the nervous system. Exp Neurobiol 22(3):149–157. https://doi.org/10.5607/en.2013.22.3.149
Chouliaras L, Mastroeni D, Delvaux E, Grover A, Kenis G, Hof PR, Steinbusch HW, Coleman PD, Rutten BP, van den Hove DL (2013) Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol Aging 34(9):2091–2099. https://doi.org/10.1016/j.neurobiolaging.2013.02.021
Deng Y, Chen D, Wang L, Gao F, Jin B, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Miao Z (2019) Silencing of long noncoding RNA nespas aggravates microglial cell death and neuroinflammation in ischemic stroke. Stroke 50(7):1850–1858. https://doi.org/10.1161/STROKEAHA.118.023376
Di Pietro A, Kajaste-Rudnitski A, Oteiza A, Nicora L, Towers GJ, Mechti N, Vicenzi E (2013) TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J Virol 87(8):4523–4533. https://doi.org/10.1128/JVI.02548-12
Dong W, Qiu C, Gong D, Jiang X, Liu W, Liu W, Zhang L, Zhang W (2019) Proteomics and bioinformatics approaches for the identification of plasma biomarkers to detect Parkinson’s disease. Exp Ther Med 18(4):2833–2842. https://doi.org/10.3892/etm.2019.7888
Dong W, Luo B, Qiu C, Jiang X, Shen B, Zhang L, Liu W, Zhang W (2020) TRIM3 attenuates apoptosis in Parkinson’s disease via activating PI3K/AKT signal pathway. Aging 13(1):735–749. https://doi.org/10.18632/aging.202181
El Husseini N, Schlisser AE, Hales BF (2016) Editor’s highlight: hydroxyurea exposure activates the P53 signaling pathway in murine organogenesis-stage embryos. Toxicol Sci 152(2):297–308. https://doi.org/10.1093/toxsci/kfw089
Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, Zhang H, Fu S, Qin J, Yang J (2010) Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem 285(8):5347–5360. https://doi.org/10.1074/jbc.M109.076976
Fan W, Wu M, Qian S, Zhou Y, Chen H, Li X, Qian P (2016) TRIM52 inhibits Japanese Encephalitis Virus replication by degrading the viral NS2A. Sci Rep 6:33698. https://doi.org/10.1038/srep33698
Fan W, Liu T, Li X, Zhou Y, Wu M, Cui X, Chen H, Qian P (2017) TRIM52: A nuclear TRIM protein that positively regulates the nuclear factor-kappa B signaling pathway. Mol Immunol 82:114–122. https://doi.org/10.1016/j.molimm.2017.01.003
Farrell K, Jarome TJ (2021) Is PROTAC technology really a game changer for central nervous system drug discovery? Expert Opin Drug Discov 16(8):833–840. https://doi.org/10.1080/17460441.2021.1915979
Fu B, Wang L, Ding H, Schwamborn JC, Li S, Dorf ME (2015) TRIM32 senses and restricts influenza a virus by ubiquitination of PB1 polymerase. PLoS Pathog 11(6):e1004960. https://doi.org/10.1371/journal.ppat.1004960
Fu Q, Zou MM, Zhu JW, Zhang Y, Chen WJ, Cheng M, Liu CF, Ma QH, Xu RX (2017) TRIM32 affects the recovery of motor function following spinal cord injury through regulating proliferation of glia. Oncotarget 8(28):45380–45390. https://doi.org/10.18632/oncotarget.17492
Gonzalez-Cano L, Hillje AL, Fuertes-Alvarez S, Marques MM, Blanch A, Ian RW, Irwin MS, Schwamborn JC, Marin MC (2013) Regulatory feedback loop between TP73 and TRIM32. Cell Death Dis 4:e704. https://doi.org/10.1038/cddis.2013.224
Guan F, Huang T, Wang X, Xing Q, Gumpper K, Li P, Song J, Tan T, Yang GL, Zang X, Zhang J, Wang Y, Yang Y, Liu Y, Zhang Y, Yang B, Ma J, Ma S (2019) The TRIM protein Mitsugumin 53 enhances survival and therapeutic efficacy of stem cells in murine traumatic brain injury. Stem Cell Res Ther 10(1):352. https://doi.org/10.1186/s13287-019-1433-4
Guo L, Giasson BI, Glavis-Bloom A, Brewer MD, Shorter J, Gitler AD, Yang X (2014) A cellular system that degrades misfolded proteins and protects against neurodegeneration. Mol Cell 55(1):15–30. https://doi.org/10.1016/j.molcel.2014.04.030
Gushchina LV, Kwiatkowski TA, Bhattacharya S, Weisleder NL (2018) Conserved structural and functional aspects of the tripartite motif gene family point towards therapeutic applications in multiple diseases. Pharmacol Ther 185:12–25. https://doi.org/10.1016/j.pharmthera.2017.10.020
Han C, Xia X, Jiao S, Li G, Ran Q, Yao S (2019) Tripartite motif containing protein 37 involves in thrombin stimulated BV-2 microglial cell apoptosis and interleukin 1beta release. Biochem Biophys Res Commun 516(4):1252–1257. https://doi.org/10.1016/j.bbrc.2019.06.158
Hansen B, Oturai AB, Harbo HF, Celius EG, Nissen KK, Laska MJ, Sondergaard HB, Petersen T, Nexo BA (2011) Genetic association of multiple sclerosis with the marker rs391745 near the endogenous retroviral locus HERV-Fc1: analysis of disease subtypes. PLoS ONE 6(10):e26438. https://doi.org/10.1371/journal.pone.0026438
Hao MQ, Xie LJ, Leng W, Xue RW (2019) Trim47 is a critical regulator of cerebral ischemia-reperfusion injury through regulating apoptosis and inflammation. Biochem Biophys Res Commun 515(4):651–657. https://doi.org/10.1016/j.bbrc.2019.05.065
Hatakeyama S (2017) TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci 42(4):297–311. https://doi.org/10.1016/j.tibs.2017.01.002
Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, Zanella F, Hilker R (2009) Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 132(Pt 12):3285–3297. https://doi.org/10.1093/brain/awp293
Hillje AL, Pavlou MA, Beckmann E, Worlitzer MM, Bahnassawy L, Lewejohann L, Palm T, Schwamborn JC (2013) TRIM32-dependent transcription in adult neural progenitor cells regulates neuronal differentiation. Cell Death Dis 4:e976. https://doi.org/10.1038/cddis.2013.487
Hristova V, Sun S, Zhang H, Chan DW (2020) Proteomic analysis of degradation ubiquitin signaling by ubiquitin occupancy changes responding to 26S proteasome inhibition. Clin Proteomics 17:2. https://doi.org/10.1186/s12014-020-9265-x
Huang Q, Zhu X, Xu M (2019) Silencing of TRIM10 alleviates apoptosis in cellular model of Parkinson’s disease. Biochem Biophys Res Commun 518(3):451–458. https://doi.org/10.1016/j.bbrc.2019.08.041
Jabbari E, Woodside J, Tan MMX, Shoai M, Pittman A, Ferrari R, Mok KY, Zhang D, Reynolds RH, de Silva R, Grimm MJ, Respondek G, Muller U, Al-Sarraj S, Gentleman SM, Lees AJ, Warner TT, Hardy J, Revesz T, Hoglinger GU, Holton JL, Ryten M, Morris HR (2018) Variation at the TRIM11 locus modifies progressive supranuclear palsy phenotype. Ann Neurol 84(4):485–496. https://doi.org/10.1002/ana.25308
Jangampalli AP (1867) Reddy PH (2021) Phosphorylated tau targeted small-molecule PROTACs for the treatment of Alzheimer’s disease and tauopathies. Biochim Biophys Acta 8:166162. https://doi.org/10.1016/j.bbadis.2021.166162
Ji B, Liu L, Guo Y, Ming F, Jiang J, Li F, Zhao G, Wen J, Li N (2021) Upregulated tripartite motif 47 could facilitate glioma cell proliferation and metastasis as a tumorigenesis promoter. Comput Math Methods Med 2021:5594973. https://doi.org/10.1155/2021/5594973
Johnson PJ, Parker SR, Sakiyama-Elbert SE (2010) Fibrin-based tissue engineering scaffolds enhance neural fiber sprouting and delay the accumulation of reactive astrocytes at the lesion in a subacute model of spinal cord injury. J Biomed Mater Res A 92(1):152–163. https://doi.org/10.1002/jbm.a.32343
Jost CA, Marin MC, Kaelin WG Jr (1997) p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 389(6647):191–194. https://doi.org/10.1038/38298
Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, McKeon F, Caput D (1997) Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90(4):809–819. https://doi.org/10.1016/s0092-8674(00)80540-1
Kalaani J, Roche J, Hamade E, Badran B, Jaber M, Gaillard A, Prestoz L (2016) Axon guidance molecule expression after cell therapy in a mouse model of Parkinson’s disease. Restor Neurol Neurosci 34(6):877–895. https://doi.org/10.3233/RNN-150587
Kang WS, Park JK, Kim YJ, Cho AR, Park HJ, Kim SK, Paik JW, Lee KJ, Na HR, Kim YY, Lim HK, Jeong HG, Kim JW (2016) Association of tripartite motif family-like 2 (TRIML2) polymorphisms with late-onset Alzheimer’s disease risk in a Korean population. Neurosci Lett 630:127–131. https://doi.org/10.1016/j.neulet.2016.07.046
Keeble AH, Khan Z, Forster A, James LC (2008) TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A 105(16):6045–6050. https://doi.org/10.1073/pnas.0800159105
Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11(8):720–731. https://doi.org/10.1016/S1474-4422(12)70104-7
Khan F, Khan TJ, Kalamegam G, Pushparaj PN, Chaudhary A, Abuzenadah A, Kumosani T, Barbour E, Al-Qahtani M (2017) Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern Med 17(1):418. https://doi.org/10.1186/s12906-017-1926-6
Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci 38(7):1167–1186. https://doi.org/10.1007/s10072-017-2938-1
Kung CP, Khaku S, Jennis M, Zhou Y, Murphy ME (2015) Identification of TRIML2, a novel p53 target, that enhances p53 SUMOylation and regulates the transactivation of proapoptotic genes. Mol Cancer Res 13(2):250–262. https://doi.org/10.1158/1541-7786.MCR-14-0385
Lambert C, Cisternas P, Inestrosa NC (2016) Role of Wnt signaling in central nervous system injury. Mol Neurobiol 53(4):2297–2311. https://doi.org/10.1007/s12035-015-9138-x
Lanni C, Uberti D, Racchi M, Govoni S, Memo M (2007) Unfolded p53: a potential biomarker for Alzheimer’s disease. J Alzheimers Dis 12(1):93–99. https://doi.org/10.3233/jad-2007-12109
Lassot I, Robbins I, Kristiansen M, Rahmeh R, Jaudon F, Magiera MM, Mora S, Vanhille L, Lipkin A, Pettmann B, Ham J, Desagher S (2010) Trim17, a novel E3 ubiquitin-ligase, initiates neuronal apoptosis. Cell Death Differ 17(12):1928–1941. https://doi.org/10.1038/cdd.2010.73
Lassot I, Mora S, Lesage S, Zieba BA, Coque E, Condroyer C, Bossowski JP, Mojsa B, Marelli C, Soulet C, Tesson C, Carballo-Carbajal I, Laguna A, Mangone G, Vila M, Brice A, Desagher S (2018) The E3 ubiquitin ligases TRIM17 and TRIM41 modulate alpha-synuclein expression by regulating ZSCAN21. Cell Rep 25(9):2484–2496. https://doi.org/10.1016/j.celrep.2018.11.002
Lee D, Hong JH (2022) Physiological overview of the potential link between the UPS and Ca(2+) signaling. Antioxidants (Basel) 11(5):997. https://doi.org/10.3390/antiox11050997
Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT (1996) Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg 84(1):91–96. https://doi.org/10.3171/jns.1996.84.1.0091
Lee JH, Cheng R, Vardarajan B, Lantigua R, Reyes-Dumeyer D, Ortmann W, Graham RR, Bhangale T, Behrens TW, Medrano M, Jimenez-Velazquez IZ, Mayeux R (2015) Genetic modifiers of age at onset in carriers of the G206A mutation in PSEN1 with familial Alzheimer disease among Caribbean Hispanics. JAMA Neurol 72(9):1043–1051. https://doi.org/10.1001/jamaneurol.2015.1424
Lepore E, Casola I, Dobrowolny G, Musaro A (2019) Neuromuscular junction as an entity of nerve-muscle communication. Cells 8(8):906. https://doi.org/10.3390/cells8080906
Li Q, Yan J, Mao AP, Li C, Ran Y, Shu HB, Wang YY (2011) Tripartite motif 8 (TRIM8) modulates TNFalpha- and IL-1beta-triggered NF-kappaB activation by targeting TAK1 for K63-linked polyubiquitination. Proc Natl Acad Sci U S A 108(48):19341–19346. https://doi.org/10.1073/pnas.1110946108
Li M, Sun M, Cao L, Gu JH, Ge J, Chen J, Han R, Qin YY, Zhou ZP, Ding Y, Qin ZH (2014) A TIGAR-regulated metabolic pathway is critical for protection of brain ischemia. J Neurosci 34(22):7458–7471. https://doi.org/10.1523/JNEUROSCI.4655-13.2014
Li C, Ou R, Hou Y, Chen Y, Gu X, Wei Q, Cao B, Zhang L, Liu K, Chen X, Song W, Zhao B, Wu Y, Shang H (2021) Genetic analysis of TRIM family genes for early-onset Parkinson’s disease in Chinese population. Parkinsonism Relat Disord 90:105–113. https://doi.org/10.1016/j.parkreldis.2021.08.005
Lin CW, Chen B, Huang KL, Dai YS, Teng HL (2016) Inhibition of autophagy by estradiol promotes locomotor recovery after spinal cord injury in rats. Neurosci Bull 32(2):137–144. https://doi.org/10.1007/s12264-016-0017-x
Liu X, Lei Q (2020) TRIM62 knockout protects against cerebral ischemic injury in mice by suppressing NLRP3-regulated neuroinflammation. Biochem Biophys Res Commun 529(2):140–147. https://doi.org/10.1016/j.bbrc.2020.06.014
Liu Y, Zhu M, Lin L, Fan X, Piao Z, Jiang X (2014) Deficiency of Trim27 protects dopaminergic neurons from apoptosis in the neurotoxin model of Parkinson’s disease. Brain Res 1588:17–24. https://doi.org/10.1016/j.brainres.2014.09.018
Liu P, Cui L, Shen L (2020) Knockdown of TRIM52 alleviates LPS-induced inflammatory injury in human periodontal ligament cells through the TLR4/NF-kappaB pathway. Biosci Rep 40(8):1223. https://doi.org/10.1042/BSR20201223
Liu L, Yang C, Lavayen BP, Tishko RJ, Larochelle J, Candelario-Jalil E (2022) Targeted BRD4 protein degradation by dBET1 ameliorates acute ischemic brain injury and improves functional outcomes associated with reduced neuroinflammation and oxidative stress and preservation of blood–brain barrier integrity. J Neuroinflamm 19(1):168. https://doi.org/10.1186/s12974-022-02533-8
Lokapally A, Neuhaus H, Herfurth J, Hollemann T (2020) Interplay of TRIM2 E3 ubiquitin ligase and ALIX/ESCRT complex: control of developmental plasticity during early neurogenesis. Cells 9(7):1734. https://doi.org/10.3390/cells9071734
Lu K, Pan Y, Huang Z, Liang H, Ding ZY, Zhang B (2022) TRIM proteins in hepatocellular carcinoma. J Biomed Sci 29(1):69. https://doi.org/10.1186/s12929-022-00854-7
Luo J, Sun L, Lin X, Liu G, Yu J, Parisiadou L, Xie C, Ding J, Cai H (2014) A calcineurin- and NFAT-dependent pathway is involved in alpha-synuclein-induced degeneration of midbrain dopaminergic neurons. Hum Mol Genet 23(24):6567–6574. https://doi.org/10.1093/hmg/ddu377
Luscher B, Larsson LG (1999) The basic region/helix-loop-helix/leucine zipper domain of Myc proto-oncoproteins: function and regulation. Oncogene 18(19):2955–2966. https://doi.org/10.1038/sj.onc.1202750
Ma Z, Zang T, Birnbaum SG, Wang Z, Johnson JE, Zhang CL, Parada LF (2017) TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun 8(1):1668. https://doi.org/10.1038/s41467-017-01709-8
Ma K, Han XX, Yang XM, Zhou SL (2021) Proteolysis targeting chimera technology: a novel strategy for treating diseases of the central nervous system. Neural Regen Res 16(10):1944–1949. https://doi.org/10.4103/1673-5374.308075
Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC (2010) Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107(46):19985–19990. https://doi.org/10.1073/pnas.1014074107
Manocha GD, Mishra R, Sharma N, Kumawat KL, Basu A, Singh SK (2014) Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J Neuroinflamm 11:24. https://doi.org/10.1186/1742-2094-11-24
McDonald JW, Becker D (2003) Spinal cord injury: promising interventions and realistic goals. Am J Phys Med Rehabil 82(10 Suppl):S38-49. https://doi.org/10.1097/01.PHM.0000086994.53716.17
McEwan WA, Falcon B, Vaysburd M, Clift D, Oblak AL, Ghetti B, Goedert M, James LC (2017) Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc Natl Acad Sci U S A 114(3):574–579. https://doi.org/10.1073/pnas.1607215114
McNab FW, Rajsbaum R, Stoye JP, O’Garra A (2011) Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol 23(1):46–56. https://doi.org/10.1016/j.coi.2010.10.021
Mendioroz Iriarte M, Pulido Fontes L, Mendez-Lopez I (2015) Neuroepigenetics: desoxyribonucleic acid methylation in Alzheimer’s disease and other dementias. Med Clin (barc) 144(10):457–464. https://doi.org/10.1016/j.medcli.2014.03.023
Meng F, Wang J, Ding F, Xie Y, Zhang Y, Zhu J (2017) Neuroprotective effect of matrine on MPTP-induced Parkinson’s disease and on Nrf2 expression. Oncol Lett 13(1):296–300. https://doi.org/10.3892/ol.2016.5383
Menon S, Boyer NP, Winkle CC, McClain LM, Hanlin CC, Pandey D, Rothenfusser S, Taylor AM, Gupton SL (2015) The E3 ubiquitin ligase TRIM9 is a filopodia off switch required for netrin-dependent axon guidance. Dev Cell 35(6):698–712. https://doi.org/10.1016/j.devcel.2015.11.022
Mojsa B, Mora S, Bossowski JP, Lassot I, Desagher S (2015) Control of neuronal apoptosis by reciprocal regulation of NFATc3 and Trim17. Cell Death Differ 22(2):274–286. https://doi.org/10.1038/cdd.2014.141
Moller T, Hanisch UK, Ransom BR (2000) Thrombin-induced activation of cultured rodent microglia. J Neurochem 75(4):1539–1547. https://doi.org/10.1046/j.1471-4159.2000.0751539.x
Monti C, Colugnat I, Lopiano L, Chio A, Alberio T (2018) Network analysis identifies disease-specific pathways for Parkinson’s disease. Mol Neurobiol 55(1):370–381. https://doi.org/10.1007/s12035-016-0326-0
Morris G, Maes M, Murdjeva M, Puri BK (2019) Do human endogenous retroviruses contribute to multiple sclerosis, and if so, how? Mol Neurobiol 56(4):2590–2605. https://doi.org/10.1007/s12035-018-1255-x
Mu X, Li H, Zhou L, Xu W (2019) TRIM52 regulates the proliferation and invasiveness of lung cancer cells via the Wnt/betacatenin pathway. Oncol Rep 41(6):3325–3334. https://doi.org/10.3892/or.2019.7110
Mullard A (2021) Targeted protein degraders crowd into the clinic. Nat Rev Drug Discov 20(4):247–250. https://doi.org/10.1038/d41573-021-00052-4
Napolitano LM, Meroni G (2012) TRIM family: pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life 64(1):64–71. https://doi.org/10.1002/iub.580
Nenasheva VV, Novosadova EV, Makarova IV, Lebedeva OS, Grefenshtein MA, Arsenyeva EL, Antonov SA, Grivennikov IA, Tarantul VZ (2017) The transcriptional changes of trim genes associated with Parkinson’s disease on a model of human induced pluripotent stem cells. Mol Neurobiol 54(9):7204–7211. https://doi.org/10.1007/s12035-016-0230-7
Nenasheva VV, Nikitenko NA, Stepanenko EA, Makarova IV, Andreeva LE, Kovaleva GV, Lysenko AA, Tukhvatulin AI, Logunov DY, Tarantul VZ (2021) Human TRIM14 protects transgenic mice from influenza A viral infection without activation of other innate immunity pathways. Genes Immun 22(1):56–63. https://doi.org/10.1038/s41435-021-00128-6
Nexo BA, Christensen T, Frederiksen J, Moller-Larsen A, Oturai AB, Villesen P, Hansen B, Nissen KK, Laska MJ, Petersen TS, Bonnesen S, Hedemand A, Wu T, Wang X, Zhang X, Brudek T, Maric R, Sondergaard HB, Sellebjerg F, Brusgaard K, Kjeldbjerg AL, Rasmussen HB, Nielsen AL, Nyegaard M, Petersen T, Borglum AD, Pedersen FS (2011) The etiology of multiple sclerosis: genetic evidence for the involvement of the human endogenous retrovirus HERV-Fc1. PLoS ONE 6(2):e16652. https://doi.org/10.1371/journal.pone.0016652
Nexo BA, Hansen B, Nissen KK, Gundestrup L, Terkelsen T, Villesen P, Bahrami S, Petersen T, Pedersen FS, Laska MJ (2013) Restriction genes for retroviruses influence the risk of multiple sclerosis. PLoS ONE 8(9):e74063. https://doi.org/10.1371/journal.pone.0074063
Niedermeyer S, Murn M, Choi PJ (2019) Respiratory failure in amyotrophic lateral sclerosis. Chest 155(2):401–408. https://doi.org/10.1016/j.chest.2018.06.035
Norris KL, Hao R, Chen LF, Lai CH, Kapur M, Shaughnessy PJ, Chou D, Yan J, Taylor JP, Engelender S, West AE, Lim KL, Yao TP (2015) Convergence of parkin, PINK1, and alpha-synuclein on stress-induced mitochondrial morphological remodeling. J Biol Chem 290(22):13862–13874. https://doi.org/10.1074/jbc.M114.634063
Ntim M, Li QF, Zhang Y, Liu XD, Li N, Sun HL, Zhang X, Khan B, Wang B, Wu Q, Wu XF, Walana W, Khan K, Ma QH, Zhao J, Li S (2020) TRIM32 deficiency impairs synaptic plasticity by excitatory-inhibitory imbalance via notch pathway. Cereb Cortex 30(8):4617–4632. https://doi.org/10.1093/cercor/bhaa064
Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ (2015) The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol 78:23–34. https://doi.org/10.1016/j.yjmcc.2014.11.005
OuYang X, Guo J, Lv Q, Jiang H, Zheng Y, Liu P, Zhao T, Kong D, Hao H, Jiang Y (2020) TRIM32 drives pathogenesis in streptococcal toxic shock-like syndrome and streptococcus suis meningitis by regulating innate immune responses. Infect Immun. https://doi.org/10.1128/IAI.00957-19
Owens DM, Keyse SM (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26(22):3203–3213. https://doi.org/10.1038/sj.onc.1210412
Ozato K, Shin DM, Chang TH, Morse HC 3rd (2008) TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol 8(11):849–860. https://doi.org/10.1038/nri2413
Perna A, Marathe S, Dreos R, Falquet L, Akarsu Egger H, Auber LA (2021) Revealing NOTCH-dependencies in synaptic targets associated with Alzheimer’s disease. Mol Cell Neurosci 115:103657. https://doi.org/10.1016/j.mcn.2021.103657
Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grutter MG, Luban J (2011) TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472(7343):361–365. https://doi.org/10.1038/nature09976
Philouze C, Turban S, Cremers B, Caliez A, Lamarche G, Bernard C, Provost N, Delerive P (2021) MG53 is not a critical regulator of insulin signaling pathway in skeletal muscle. PLoS ONE 16(2):e0245179. https://doi.org/10.1371/journal.pone.0245179
Ponten F, Jirstrom K, Uhlen M (2008) The Human Protein Atlas–a tool for pathology. J Pathol 216(4):387–393. https://doi.org/10.1002/path.2440.HumanProteinAtlasproteinatlas.org
Randolph K, Hyder U, D’Orso I (2022) KAP1/TRIM28: transcriptional activator and/or repressor of viral and cellular programs? Front Cell Infect Microbiol 12:834636. https://doi.org/10.3389/fcimb.2022.834636
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A (2001) The tripartite motif family identifies cell compartments. EMBO J 20(9):2140–2151. https://doi.org/10.1093/emboj/20.9.2140
Rousseaux MW, de Haro M, Lasagna-Reeves CA, De Maio A, Park J, Jafar-Nejad P, Al-Ramahi I, Sharma A, See L, Lu N, Vilanova-Velez L, Klisch TJ, Westbrook TF, Troncoso JC, Botas J, Zoghbi HY (2016) TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife. https://doi.org/10.7554/eLife.19809
Rousseaux MW, Revelli JP, Vazquez-Velez GE, Kim JY, Craigen E, Gonzales K, Beckinghausen J, Zoghbi HY (2018) Depleting Trim28 in adult mice is well tolerated and reduces levels of alpha-synuclein and tau. Elife. https://doi.org/10.7554/eLife.36768
Sajadimajd S, Yazdanparast R, Roshanzamir F (2016) Augmentation of oxidative stress-induced apoptosis in MCF7 cells by ascorbate-tamoxifen and/or ascorbate-juglone treatments. In Vitro Cell Dev Biol Anim 52(2):193–203. https://doi.org/10.1007/s11626-015-9961-4
Salter MW, Stevens B (2017) Microglia emerge as central players in brain disease. Nat Med 23(9):1018–1027. https://doi.org/10.1038/nm.4397
Samanta S, Perkinton MS, Morgan M, Williams RJ (1998) Hydrogen peroxide enhances signal-responsive arachidonic acid release from neurons: role of mitogen-activated protein kinase. J Neurochem 70(5):2082–2090. https://doi.org/10.1046/j.1471-4159.1998.70052082.x
Schmidt F, Kny M, Zhu X, Wollersheim T, Persicke K, Langhans C, Lodka D, Kleber C, Weber-Carstens S, Fielitz J (2014) The E3 ubiquitin ligase TRIM62 and inflammation-induced skeletal muscle atrophy. Crit Care 18(5):545. https://doi.org/10.1186/s13054-014-0545-6
Schrag A, Ben-Shlomo Y, Quinn NP (1999) Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 354(9192):1771–1775. https://doi.org/10.1016/s0140-6736(99)04137-9
Schwamborn JC, Berezikov E, Knoblich JA (2009) The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 136(5):913–925. https://doi.org/10.1016/j.cell.2008.12.024
Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR (2000) Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14(19):2501–2514. https://doi.org/10.1101/gad.836800
Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6(11):1054–1061. https://doi.org/10.1038/ncb1104-1054
Shi M, Cho H, Inn KS, Yang A, Zhao Z, Liang Q, Versteeg GA, Amini-Bavil-Olyaee S, Wong LY, Zlokovic BV, Park HS, Garcia-Sastre A, Jung JU (2014) Negative regulation of NF-kappaB activity by brain-specific TRIpartite Motif protein 9. Nat Commun 5:4820. https://doi.org/10.1038/ncomms5820
Short KM, Cox TC (2006) Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem 281(13):8970–8980. https://doi.org/10.1074/jbc.M512755200
Sun S, Hu F, Wu J, Zhang S (2017) Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol 11:577–585. https://doi.org/10.1016/j.redox.2016.12.029
Tanji K, Kamitani T, Mori F, Kakita A, Takahashi H, Wakabayashi K (2010) TRIM9, a novel brain-specific E3 ubiquitin ligase, is repressed in the brain of Parkinson’s disease and dementia with Lewy bodies. Neurobiol Dis 38(2):210–218. https://doi.org/10.1016/j.nbd.2010.01.007
Taylor RT, Lubick KJ, Robertson SJ, Broughton JP, Bloom ME, Bresnahan WA, Best SM (2011) TRIM79alpha, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 10(3):185–196. https://doi.org/10.1016/j.chom.2011.08.004
Trevisan AC, Ribeiro FB, Itikawa EN, Alexandre LS, Pitella FA, Santos AC, Simoes BP, Wichert-Ana L (2017) 18F-FDG PET/CT/MRI fusion images showing cranial and peripheral nerve involvement in neurolymphomatosis. Indian J Nucl Med 32(1):77–78. https://doi.org/10.4103/0972-3919.198502
Trono D (2015) Transposable elements, polydactyl proteins, and the genesis of human-specific transcription networks. Cold Spring Harb Symp Quant Biol 80:281–288. https://doi.org/10.1101/sqb.2015.80.027573
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS (2022) Heart disease and stroke statistics-2022 update: A report from the American Heart Association. Circulation 145(8):e153–e639. https://doi.org/10.1161/CIR.0000000000001052
Unni SK, Ruzek D, Chhatbar C, Mishra R, Johri MK, Singh SK (2011) Japanese encephalitis virus: from genome to infectome. Microbes Infect 13(4):312–321. https://doi.org/10.1016/j.micinf.2011.01.002
Valentino RR, Koga S, Heckman MG, Brushaber DE, Diehl NN, Walton RL, Dickson DW, Ross OA (2020) Association of tripartite motif containing 11 rs564309 with tau pathology in progressive supranuclear palsy. Mov Disord 35(5):890–894. https://doi.org/10.1002/mds.28010
von Grabowiecki Y, Abreu P, Blanchard O, Palamiuc L, Benosman S, Meriaux S, Devignot V, Gross I, Mellitzer G, Gonzalez de Aguilar JL, Gaiddon C (2016) Transcriptional activator TAp63 is upregulated in muscular atrophy during ALS and induces the pro-atrophic ubiquitin ligase Trim63. Elife. https://doi.org/10.7554/eLife.10528
Wang X, Hai C (2016) Novel insights into redox system and the mechanism of redox regulation. Mol Biol Rep 43(7):607–628. https://doi.org/10.1007/s11033-016-4022-y
Wang P, Benhenda S, Wu H, Lallemand-Breitenbach V, Zhen T, Jollivet F, Peres L, Li Y, Chen SJ, Chen Z, de The H, Meng G (2018) RING tetramerization is required for nuclear body biogenesis and PML sumoylation. Nat Commun 9(1):1277. https://doi.org/10.1038/s41467-018-03498-0
Wang B, Wang G, Wang Q, Zhu Z, Wang Y, Chen K, Yang H (2019) Silencing of TRIM11 suppresses the tumorigenicity of chordoma cells through improving the activity of PHLPP1/AKT. Cancer Cell Int 19:284. https://doi.org/10.1186/s12935-019-1007-7
Wang W, Zhou Q, Jiang T, Li S, Ye J, Zheng J, Wang X, Liu Y, Deng M, Ke D, Wang Q, Wang Y, Wang JZ (2021) A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics 11(11):5279–5295. https://doi.org/10.7150/thno.55680
Wei L, Zhang JS, Ji SF, Xu H, Zhao ZH, Zhang L, Pang L, Zhang JF, Yang PB, Ma H (2019) Knockdown of TRIM32 protects hippocampal neurons from oxygen-glucose deprivation-induced injury. Neurochem Res 44(9):2182–2189. https://doi.org/10.1007/s11064-019-02857-7
Wen Z, Zhang J, Tang P, Tu N, Wang K, Wu G (2018) Overexpression of miR185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol Med Rep 17(1):131–137. https://doi.org/10.3892/mmr.2017.7897
Wezyk M, Spolnicka M, Pospiech E, Peplonska B, Zbiec-Piekarska R, Ilkowski J, Styczynska M, Barczak A, Zboch M, Filipek-Gliszczynska A, Skrzypczak M, Ginalski K, Kabza M, Makalowska I, Barcikowska-Kotowicz M, Branicki W, Zekanowski C (2018a) Hypermethylation of TRIM59 and KLF14 influences cell death signaling in familial Alzheimer’s disease. Oxid Med Cell Longev 2018:6918797. https://doi.org/10.1155/2018/6918797
Wezyk M, Szybinska A, Wojsiat J, Szczerba M, Day K, Ronnholm H, Kele M, Berdynski M, Peplonska B, Fichna JP, Ilkowski J, Styczynska M, Barczak A, Zboch M, Filipek-Gliszczynska A, Bojakowski K, Skrzypczak M, Ginalski K, Kabza M, Makalowska I, Barcikowska-Kotowicz M, Wojda U, Falk A, Zekanowski C (2018b) Overactive BRCA1 Affects Presenilin 1 in Induced Pluripotent Stem Cell-Derived Neurons in Alzheimer’s Disease. J Alzheimers Dis 62(1):175–202. https://doi.org/10.3233/JAD-170830
Witoelar A, Jansen IE, Wang Y, Desikan RS, Gibbs JR, Blauwendraat C, Thompson WK, Hernandez DG, Djurovic S, Schork AJ, Bettella F, Ellinghaus D, Franke A, Lie BA, McEvoy LK, Karlsen TH, Lesage S, Morris HR, Brice A, Wood NW, Heutink P, Hardy J, Singleton AB, Dale AM, Gasser T, Andreassen OA, Sharma M, International Parkinson’s Disease Genomics Consortium NABEC, United Kingdom Brain Expression Consortium I (2017) Genome-wide pleiotropy between parkinson disease and autoimmune diseases. JAMA Neurol 74(7):780–792. https://doi.org/10.1001/jamaneurol.2017.0469
Xie H, Wang Y, Zhu T, Feng S, Yan Z, Zhu Z, Ni J, Ni J, Du R, Zhu J, Ding F, Liu S, Han H, Zhang H, Zhao J, Zhang R, Quan W, Yan X (2020) Serum MG53/TRIM72 is associated with the presence and severity of coronary artery disease and acute myocardial infarction. Front Physiol 11:617845. https://doi.org/10.3389/fphys.2020.617845
Xie X, Wang F, Li X (2021) Inhibition of TRIM14 protects cerebral ischemia/reperfusion injury through regulating NF-kappaB/NLRP3 pathway-mediated inflammation and apoptosis. J Recept Signal Transduct Res. https://doi.org/10.1080/10799893.2021.1887218
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14(2):128–142. https://doi.org/10.1038/nrn3407
Xue W, Zhao Y, Xiao Z, Wu X, Ma D, Han J, Li X, Xue X, Yang Y, Fang Y, Fan C, Liu S, Xu B, Han S, Chen B, Zhang H, Fan Y, Liu W, Dong Q, Dai J (2020) Epidermal growth factor receptor-extracellular-regulated kinase blockade upregulates TRIM32 signaling cascade and promotes neurogenesis after spinal cord injury. Stem Cells 38(1):118–133. https://doi.org/10.1002/stem.3097
Yalcinkaya N, Haytural H, Bilgic B, Ozdemir O, Hanagasi H, Kucukali CI, Ozbek Z, Akcan U, Idrisoglu HA, Gurvit H, Tuzun E (2016) Expression changes of genes associated with apoptosis and survival processes in Parkinson’s disease. Neurosci Lett 615:72–77. https://doi.org/10.1016/j.neulet.2016.01.029
Yang K, Shi HX, Liu XY, Shan YF, Wei B, Chen S, Wang C (2009) TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J Immunol 182(6):3782–3792. https://doi.org/10.4049/jimmunol.0803126
Yang T, Hu Y, Miao J, Chen J, Liu J, Cheng Y, Gao X (2022) A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation, apoptosis and M2 macrophages polarization. Acta Pharm Sin B 12(6):2658–2671. https://doi.org/10.1016/j.apsb.2022.02.009
Yao Y, Zhang B, Zhu H, Li H, Han Y, Chen K, Wang Z, Zeng J, Liu Y, Wang X, Li Y, He D, Lin P, Zhou X, Park KH, Bian Z, Chen Z, Gong N, Tan T, Zhou J, Zhang M, Ma J, Zeng C (2016) MG53 permeates through blood–brain barrier to protect ischemic brain injury. Oncotarget 7(16):22474–22485. https://doi.org/10.18632/oncotarget.7965
Yi J, Li A, Li X, Park K, Zhou X, Yi F, Xiao Y, Yoon D, Tan T, Ostrow LW, Ma J, Zhou J (2021) MG53 preserves neuromuscular junction integrity and alleviates ALS disease progression. Antioxidants (Basel) 10(10):1522. https://doi.org/10.3390/antiox10101522
Yokota T, Mishra M, Akatsu H, Tani Y, Miyauchi T, Yamamoto T, Kosaka K, Nagai Y, Sawada T, Heese K (2006) Brain site-specific gene expression analysis in Alzheimer’s disease patients. Eur J Clin Invest 36(11):820–830. https://doi.org/10.1111/j.1365-2362.2006.01722.x
Zeng J, Wang Y, Luo Z, Chang LC, Yoo JS, Yan H, Choi Y, Xie X, Deverman BE, Gradinaru V, Gupton SL, Zlokovic BV, Zhao Z, Jung JU (2019) TRIM9-mediated resolution of neuroinflammation confers neuroprotection upon ischemic stroke in mice. Cell Rep 27(2):549–560. https://doi.org/10.1016/j.celrep.2018.12.055
Zeng S, Zhao Z, Zheng S, Wu M, Song X, Li Y, Zheng Y, Liu B, Chen L, Gao C, Liu H (2021) The E3 ubiquitin ligase TRIM31 is involved in cerebral ischemic injury by promoting degradation of TIGAR. Redox Biol 45:102058. https://doi.org/10.1016/j.redox.2021.102058
Zhang J, Hu MM, Wang YY, Shu HB (2012) TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem 287(34):28646–28655. https://doi.org/10.1074/jbc.M112.362608
Zhang ZB, Xiong LL, Lu BT, Zhang HX, Zhang P, Wang TH (2017) Suppression of Trim32 enhances motor function repair after traumatic brain injury associated with antiapoptosis. Cell Transplant 26(7):1276–1285. https://doi.org/10.1177/0963689717716510
Zhang HM, Liu P, Jiang C, Jin XQ, Liu RN, Li SQ, Zhao Y (2018) Notch signaling inhibitor DAPT provides protection against acute craniocerebral injury. PLoS ONE 13(2):e0193037. https://doi.org/10.1371/journal.pone.0193037
Zhang JR, Li XX, Hu WN, Li CY (2020a) Emerging role of TRIM family proteins in cardiovascular disease. Cardiology 145(6):390–400. https://doi.org/10.1159/000506150
Zhang X, Feng Y, Li J, Zheng L, Shao Y, Zhu F, Sun X (2020b) MicroRNA-665–3p attenuates oxygen-glucose deprivation-evoked microglial cell apoptosis and inflammatory response by inhibiting NF-kappaB signaling via targeting TRIM8. Int Immunopharmacol 85:106650. https://doi.org/10.1016/j.intimp.2020.106650
Zhang X, Pavlicev M, Jones HN, Muglia LJ (2020c) Eutherian-specific gene TRIML2 attenuates inflammation in the evolution of placentation. Mol Biol Evol 37(2):507–523. https://doi.org/10.1093/molbev/msz238
Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH, Gao WQ (2014) TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology 147(5):1043–1054. https://doi.org/10.1053/j.gastro.2014.07.021
Zhu G, Harischandra DS, Ghaisas S, Zhang P, Prall W, Huang L, Maghames C, Guo L, Luna E, Mack KL, Torrente MP, Luk KC, Shorter J, Yang X (2020) TRIM11 prevents and reverses protein aggregation and rescues a mouse model of Parkinson’s disease. Cell Rep 33(9):108418. https://doi.org/10.1016/j.celrep.2020.108418
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province of China (Program No. BK202013 28) and the Natural Science Foundation of China (Program No. 82073845, 82174051).
Author information
Authors and Affiliations
Contributions
MP contributed to conception of the study, acquisition of data and analysis, and drafting the article. XL contributed to conception of the study and revising article critically. GX and XT contributed to revising article critically. YL contributed to study supervision. WF contributed to review, revision of the manuscript, and study supervision. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, M., Li, X., Xu, G. et al. Tripartite Motif Protein Family in Central Nervous System Diseases. Cell Mol Neurobiol 43, 2567–2589 (2023). https://doi.org/10.1007/s10571-023-01337-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-023-01337-5