Abstract
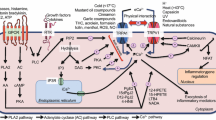
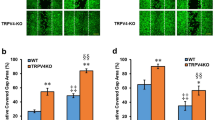
Transient receptor potential vanilloid 3 (TRPV3) is highly expressed in skin keratinocytes where it forms Ca2+-permeable nonselective cation channels to regulate various cutaneous functions. TRPV3 expression is upregulated in many skin disorders. Here, we examined how TRPV3 affects keratinocyte proliferation and investigated the underlying mechanism. Topical application of TRPV3 agonist, carvacrol, increased skin thickness in wild type (WT) mice but not in TRPV3 knockout (KO) mice. Carvacrol promoted proliferation of human keratinocytes HaCaT cells at concentrations ≤ 100 μM, but at 300 μM, it decreased cell viability, suggesting a nonmonotonic proliferative effect. Suppression of TRPV3 expression abolished carvacrol-induced cell proliferation while overexpression of TRPV3 enhanced HaCaT cell proliferation. Carvacrol also stimulated Ca2+ influx and proliferation of primary keratinocytes prepared from WT but not TRPV3 KO mice, suggesting that carvacrol-stimulated cell proliferation was dependent on TRPV3-mediated Ca2+ influx. Mechanistic investigation demonstrated that carvacrol stimulated TGFα release and increased phosphorylation levels of EGFR, PI3K, and NF-κB, effects abolished by suppression of TRPV3 expression and CaMKII inhibition. Moreover, inhibition of CaMKII, EGFR, PI3K, or NF-κB diminished carvacrol-induced cell proliferation. We conclude that while strong activation of TRPV3 may cause cell death, moderate activation of TRPV3 promotes cell proliferation in keratinocytes through Ca2+/CaMKII→TGFα/EGFR→PI3K→NF-κB signaling.

Headlights
1. Carvacrol induces epidermal hyperplasia and keratinocyte proliferation.
2. TRPV3 mediates carvacrol-induced epidermal hyperplasia and keratinocyte proliferation.
3. TRPV3 acts through Ca2+/CaMKII→TGFα/EGFR→PI3K→NF-κB signaling to promote keratinocyte proliferation.








Similar content being viewed by others
Abbreviations
- 2-APB:
-
2-Aminoethoxydiphenyl borate
- BrdU:
-
5-Bromo-2-deoxyUridine
- BSA:
-
Bovine serum albumin
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- EGFR:
-
Epidermal growth factor receptor
- EGTA:
-
Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- FBS:
-
Fetal bovine serum
- GOF:
-
Gain-of-function
- H&E:
-
Hematoxylin and eosin
- Na2EDTA:
-
Ethylenediaminetetraacetic acid disodium salt dihydrate
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- OS:
-
Olmsted Syndrome
- PI3K:
-
Phosphoinositide 3-kinase
- TGFα:
-
Transforming growth factor-α
- TRPV3:
-
Transient receptor potential vanilloid 3
- WT:
-
Wild type
References
Aijima R, Wang B, Takao T, Mihara H, Kashio M, Ohsaki Y, et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015;29(1):182–92. https://doi.org/10.1096/fj.14-251314.
Asakawa M, Yoshioka T, Hikita I, Matsutani T, Hirasawa T, Arimura A, et al. WBN/Kob-Ht rats spontaneously develop dermatitis under conventional conditions: another possible model for atopic dermatitis. Exp Anim. 2005;54(5):461–5. https://doi.org/10.1538/expanim.54.461.
Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126(12):2664–72. https://doi.org/10.1038/sj.jid.5700468.
Borbiro I, Lisztes E, Toth BI, Czifra G, Olah A, Szollosi AG, et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J Investig Dermatol. 2011;131(8):1605–14. https://doi.org/10.1038/jid.2011.122.
Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141(2):331–43. https://doi.org/10.1016/j.cell.2010.03.013.
Chung MK, Guler AD, Caterina MJ. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J Biol Chem. 2005;280(16):15928–41. https://doi.org/10.1074/jbc.M500596200.
Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–24. https://doi.org/10.1038/nature02196.
Cui TT, Wang GX, Wei NN, Wang K. A pivotal role for the activation of TRPV3 channel in itch sensations induced by the natural skin sensitizer carvacrol. Acta Pharmacol Sin. 2018;39(3):331–5. https://doi.org/10.1038/aps.2017.152.
De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf). 2012;204(2):255–66. https://doi.org/10.1111/j.1748-1716.2011.02338.x.
El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27(2):225–31. https://doi.org/10.1093/carcin/bgi220.
Feketa VV, Marrelli SP. Systemic administration of the TRPV3 ion channel agonist carvacrol induces hypothermia in conscious rodents. PLoS One. 2015;10(11):e0141994. https://doi.org/10.1371/journal.pone.0141994.
Greco C, Leclerc-Mercier S, Chaumon S, Doz F, Hadj-Rabia S, Molina T, et al. Use of epidermal growth factor receptor inhibitor erlotinib to treat palmoplantar keratoderma in patients with olmsted syndrome caused by TRPV3 mutations. JAMA Dermatol. 2020:e194126. https://doi.org/10.1001/jamadermatol.2019.4126.
Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279(34):35741–8. https://doi.org/10.1074/jbc.M404164200.
Imura K, Yoshioka T, Hirasawa T, Sakata T. Role of TRPV3 in immune response to development of dermatitis. J Inflamm (Lond). 2009;6:17. https://doi.org/10.1186/1476-9255-6-17.
Iordanov MS, Choi RJ, Ryabinina OP, Dinh TH, Bright RK, Magun BE. The UV (Ribotoxic) stress response of human keratinocytes involves the unexpected uncoupling of the Ras-extracellular signal-regulated kinase signaling cascade from the activated epidermal growth factor receptor. Mol Cell Biol. 2002;22(15):5380–94. https://doi.org/10.1128/mcb.22.15.5380-5394.2002.
Kemény Á, Kodji X, Horváth S, Komlódi R, Szőke É, Sándor Z, et al. TRPA1 acts in a protective manner in imiquimod-induced psoriasiform dermatitis in mice. J Investig Dermatol. 2018;138(8):1774–84. https://doi.org/10.1016/j.jid.2018.02.040.
Kim HO, Cho YS, Park SY, Kwak IS, Choi MG, Chung BY, et al. Increased activity of TRPV3 in keratinocytes in hypertrophic burn scars with postburn pruritus. Wound Repair Regen. 2016;24(5):841–50. https://doi.org/10.1111/wrr.12469.
Lee CW, Lin CC, Lin WN, Liang KC, Luo SF, Wu CB, et al. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L799–812. https://doi.org/10.1152/ajplung.00311.2006.
Lee SP, Buber MT, Yang Q, Cerne R, Cortés RY, Sprous DG, et al. Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol. 2008;153(8):1739–49. https://doi.org/10.1038/bjp.2008.85.
Li W, Wang H, Kuang CY, Zhu JK, Yu Y, Qin ZX, et al. An essential role for the Id1/PI3K/Akt/NFkB/survivin signalling pathway in promoting the proliferation of endothelial progenitor cells in vitro. Mol Cell Biochem. 2012;363(1–2):135–45. https://doi.org/10.1007/s11010-011-1166-x.
Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3(5):799–810. https://doi.org/10.1038/nprot.2008.50.
Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90(3):558–64. https://doi.org/10.1016/j.ajhg.2012.02.006.
Martin LJ, Smith SB, Khoutorsky A, Magnussen CA, Samoshkin A, Sorge RE, et al. Epiregulin and EGFR interactions are involved in pain processing. J Clin Invest. 2017;127(9):3353–66. https://doi.org/10.1172/jci87406.
Mascia F, Denning M, Kopan R, Yuspa SH. The black box illuminated: signals and signaling. J Investig Dermatol. 2012;132(3 Pt 2):811–9. https://doi.org/10.1038/jid.2011.406.
Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat Commun. 2011;2:369. https://doi.org/10.1038/ncomms1371.
Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Investig Dermatol. 2008;128(6):1365–74. https://doi.org/10.1038/sj.jid.5701184.
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296(5575):2046–9. https://doi.org/10.1126/science.1073140.
Peus D, Vasa RA, Meves A, Beyerle A, Pittelkow MR. UVB-induced epidermal growth factor receptor phosphorylation is critical for downstream signaling and keratinocyte survival. Photochem Photobiol. 2000;72(1):135–40. https://doi.org/10.1562/0031-8655(2000)072<0135:uiegfr>2.0.co;2.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308. https://doi.org/10.1038/nprot.2013.143.
Shen L, Yang Q, He Y, Zou X, Cao Z. BmK NT1-induced neurotoxicity is mediated by PKC/CaMKII-dependent ERK1/2 and p38 activation in primary cultured cerebellar granule cells. Toxicology. 2019;421:22–9. https://doi.org/10.1016/j.tox.2019.03.012.
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418(6894):186–90. https://doi.org/10.1038/nature00894.
Sulk M, Seeliger S, Aubert J, Schwab VD, Cevikbas F, Rivier M, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Investig Dermatol. 2012;132(4):1253–62. https://doi.org/10.1038/jid.2011.424.
Sun HZ, Yang TW, Zang WJ, Wu SF. Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. J Endocrinol. 2010;204(3):311–8. https://doi.org/10.1677/JOE-09-0270.
Sun XY, Sun LL, Qi H, Gao Q, Wang GX, Wei NN, et al. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol Pharmacol. 2018;94(4):1164–73. https://doi.org/10.1124/mol.118.112466.
Sun X, Qi H, Wu H, Qu Y, Wang K. Anti-pruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca2+-permeable TRPV3 channel. J Dermatol Sci. 2020:S0923–1811(20)30023–2. https://doi.org/10.1016/j.jdermsci.2020.01.004.
Szollosi AG, Vasas N, Angyal A, Kistamas K, Nanasi PP, Mihaly J, et al. Activation of TRPV3 regulates inflammatory actions of human epidermal keratinocytes. J Investig Dermatol. 2018;138(2):365–74. https://doi.org/10.1016/j.jid.2017.07.852.
Um JY, Kang SY, Kim HJ, Chung BY, Park CW, Kim HO. Transient receptor potential vanilloid-3 (TRPV3) channel induces dermal fibrosis via the TRPV3/TSLP/Smad2/3 pathways in dermal fibroblasts. J Dermatol Sci. 2020;97(2):117–24. https://doi.org/10.1016/j.jdermsci.2019.12.011.
Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151(4):530–40. https://doi.org/10.1038/sj.bjp.0707245.
Vriens J, Nilius B, Vennekens R. Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol. 2008;6(1):79–96. https://doi.org/10.2174/157015908783769644.
Wang G, Wang K. The Ca2+-permeable cation transient receptor potential TRPV3 channel: an emerging pivotal target for itch and skin diseases. Mol Pharmacol. 2017;92(3):193–200. https://doi.org/10.1124/mol.116.107946.
Xiao R, Tian J, Tang J, Zhu MX. The TRPV3 mutation associated with the hairless phenotype in rodents is constitutively active. Cell Calcium. 2008;43(4):334–43. https://doi.org/10.1016/j.ceca.2007.06.004.
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418(6894):181–6. https://doi.org/10.1038/nature00882.
Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9(5):628–35. https://doi.org/10.1038/nn1692.
Yamamoto-Kasai E, Yasui K, Shichijo M, Sakata T, Yoshioka T. Impact of TRPV3 on the development of allergic dermatitis as a dendritic cell modulator. Exp Dermatol. 2013;22(12):820–4. https://doi.org/10.1111/exd.12273.
Yan K, Sun X, Wang G, Liu Y, Wang K. Pharmacological activation of thermo-transient receptor potential vanilloid 3 channels inhibits hair growth by inducing cell death of hair follicle outer root sheath. J Pharmacol Exp Ther. 2019;370(2):299–307. https://doi.org/10.1124/jpet.119.258087.
Yang G, Ma H, Wu Y, Zhou B, Zhang C, Chai C, et al. Activation of TRPC6 channels contributes to (+)-conocarpan-induced apoptotic cell death in HK-2 cells. Food Chem Toxicol. 2019;129:281–90. https://doi.org/10.1016/j.fct.2019.04.061.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. https://doi.org/10.1038/35052073.
Zhang Q, Cao Y, Luo Q, Wang P, Shi P, Song C, et al. The transient receptor potential vanilloid-3 regulates hypoxia-mediated pulmonary artery smooth muscle cells proliferation via PI3K/AKT signaling pathway. Cell Prolif. 2018;51(3):e12436. https://doi.org/10.1111/cpr.12436.
Zhao J, Munanairi A, Liu X-Y, Zhang J, Hu L, Hu M, et al. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J Investig Dermatol. 2020:S0022-202X(20)30124-X. https://doi.org/10.1016/j.jid.2020.01.012.
Zheng J, Yu Y, Feng W, Li J, Liu J, Zhang C, et al. Influence of nanomolar deltamethrin on the hallmarks of primary cultured cortical neuronal network and the role of ryanodine receptors. Environ Health Perspect. 2019;127(6):67003. https://doi.org/10.1289/ehp4583.
Zhou Y, Han D, Follansbee T, Wu X, Yu S, Wang B, et al. Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced, psoriasiform dermal inflammation in mice. J Cell Mol Med. 2019;23(7):4819–28. https://doi.org/10.1111/jcmm.14392.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (81972960, 21777192); National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2018ZX09101003-004-002); and the National Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZCX201825), the “Double First-Class” project by China Pharmaceutical University (CPU2018GY18), and the joint project between China Pharmaceutical University and East South University (2242019K3DZ01).
Author information
Authors and Affiliations
Contributions
Zhengyu Cao and Fang Zhao did the conceptualization. Yujing Wang, Hang Li, and Fang Zhao were responsible for the investigation. Chu Xue, Hao Chen, and Yanning Xue contributed the data analysis. Yujing Wang, Fang Zhao, and Zhengyu Cao contributed in the writing—original draft preparation. Zhengyu Cao and Michael X. Zhu contributed in the writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 190 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Li, H., Xue, C. et al. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol Toxicol 37, 313–330 (2021). https://doi.org/10.1007/s10565-020-09536-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09536-2