Abstract
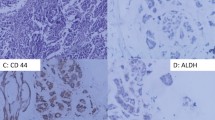
A CD44−/CD24+ phenotype is a poor prognostic marker in early invasive breast cancer. Breast cancer cells with high CD44 and low or absent CD24 (i.e. CD44+CD24−/low phenotype) are reported to have stem cell features. However, the clinical impact of CD24 and CD44 expression in tumours remains unclear. To explore the immunohistochemical expression of CD44 and CD24 (individually and combined) and their clinical value as prognostic and predictive markers. Immunohistochemical expression of CD24 and CD44 was studied in a large series of early primary invasive breast cancer tumours (n = 1036) prepared as a tissue microarray. Associations between the expression of each marker individually and in combination and clinico-pathological, molecular variables and patients’ outcome were investigated. CD24 cytoplasmic expression was significantly associated with poor prognostic variables including high tumour grade, ER−, PR−, HER2+, p53+ and triple negative (TN) phenotype; P < 0.05. However, CD24 expression was not significantly associated with patients’ outcome. Conversely, CD44 expression was associated with favourable prognostic criteria including lower Nottingham prognostic index, ER+, HER2− and luminal phenotype; P < 0.05. Moreover, CD44 expression was found to be an independent predictor of good prognosis. In combination, the CD44+/CD24− phenotype was associated with the most favourable outcome (84 and 80% 10 year breast cancer survival [BCSS] and metastasis free survival [MFS], respectively). Contrasting this, the CD44−/CD24+ phenotype was associated with the most dismal outcome (62 and 60% 10 years BCSS and MFS, respectively). CD24 and CD44 expression can individually yield prognostic data in breast cancer, but importantly, when both markers are considered; the CD44+/CD24− phenotype had the best prognosis, while the CD44−/CD24+ phenotype had the worst prognosis. This shows that the relationship between basic cell biology and clinical behaviour is not always straightforward and warrants further investigations of the true clinical impact of breast cancer stem cells.





Similar content being viewed by others
Abbreviations
- AR:
-
Androgen receptor
- BC:
-
Breast cancer
- BCSS:
-
Breast cancer-specific survival
- BLBC:
-
Basal-like breast cancer
- CK:
-
Cytokeratin
- CSC:
-
Cancer stem cells
- DAB:
-
3,3′-Diaminobenzidine
- EGFR:
-
Epidermal growth factor receptor
- ECL:
-
Enhanced chemiluminescence
- ER:
-
Oestrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- KD:
-
Kilodalton
- LR:
-
Log rank
- MFS:
-
Metastasis free survival
- NPI:
-
Nottingham prognostic index
- PBS:
-
Phosphate buffered saline
- PR:
-
Progesterone receptor
- REMARK:
-
Reporting recommendations for tumour marker prognostic studies
- TBS:
-
Tris-buffered saline
- TMA:
-
Tissue microarray
- TN:
-
Triple negative
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Dalerba P, Cho RW, Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58:267–284. doi:10.1146/annurev.med.58.062105.204854
Marx J (2003) Cancer research mutant stem cells may seed cancer. Science 301(5638):1308–1310. doi:10.1126/science.301.5638.1308
Li W, Liu F, Lei T, Xu X, Liu B, Cui L, Wei J, Guo X, Lang R, Fan Y, Gu F, Tang P, Zhang X, Fu L (2010) The clinicopathological significance of CD44+/CD24−/low and CD24+ tumor cells in invasive micropapillary carcinoma of the breast. Pathol Res Pract 206(12):828–834. doi:10.1016/j.prp.2010.09.008
Croker AK, Allan AL (2008) Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med 12(2):374–390. doi:10.1111/j.1582-4934.2007.00211.x
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988. doi:10.1073/pnas.0530291100
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG (2005) Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65(13):5506–5511. doi:10.1158/0008-5472.CAN-05-0626
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100(9):672–679. doi:10.1093/jnci/djn123
Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L (2008) The clinicopathologic and prognostic significance of CD44+/CD24(−/low) and CD44−/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol 39(7):1096–1102. doi:10.1016/j.humpath.2007.12.003
Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H (2005) Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 11(3):1154–1159
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97(16):1180–1184
Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JFR, Macmillan D, Blamey RW, Ellis IO (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116(3):340–350
Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, El-Sayed ME, Benhasouna A, Brunet JS, Akslen LA, Evans AJ, Blamey R, Reis-Filho JS, Foulkes WD, Ellis IO (2009) Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res 15(7):2302–2310. doi:10.1158/1078-0432.CCR-08-2132
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10(16):5367–5374
Ahmed MA, Jackson D, Seth R, Robins A, Lobo DN, Tomlinson IP, Ilyas M (2010) CD24 is upregulated in inflammatory bowel disease and stimulates cell motility and colony formation. Inflamm Bowel Dis 16(5):795–803. doi:10.1002/ibd.21134
Kristiansen G, Machado E, Bretz N, Rupp C, Winzer KJ, Konig AK, Moldenhauer G, Marme F, Costa J, Altevogt P (2010) Molecular and clinical dissection of CD24 antibody specificity by a comprehensive comparative analysis. Lab Invest 90(7):1102–1116. doi:10.1038/labinvest.2010.70
McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109(8):716–721
Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, Nicholson RI, Lee AH, Robertson JF, Ellis IO (2007) Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol 25(30):4772–4778
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-Tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259. doi:10.1158/1078-0432.ccr-04-0713
Ahmed MA, Al-Attar A, Kim J, Watson NF, Scholefield JH, Durrant LG, Ilyas M (2009) CD24 shows early upregulation and nuclear expression but is not a prognostic marker in colorectal cancer. J Clin Pathol 62(12):1117–1122. doi:10.1136/jcp.2009.069310
Bernard-Marty C, Cardoso F, Piccart MJ (2004) Facts and controversies in systemic treatment of metastatic breast cancer. Oncologist 9(6):617–632. doi:10.1634/theoncologist.9-6-617
Roche H, Vahdat LT (2011) Treatment of metastatic breast cancer: second line and beyond. Ann Oncol 22(5):1000–1010. doi:10.1093/annonc/mdq429
Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schluns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H, Dietel M (2003) CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res 9(13):4906–4913
Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA (2005) CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res 65(15):6755–6763. doi:10.1158/0008-5472.CAN-05-0863
Diaz LK, Zhou X, Wright ET, Cristofanilli M, Smith T, Yang Y, Sneige N, Sahin A, Gilcrease MZ (2005) CD44 expression is associated with increased survival in node-negative invasive breast carcinoma. Clin Cancer Res 11(9):3309–3314. doi:10.1158/1078-0432.CCR-04-2184
Bankfalvi A, Terpe HJ, Breukelmann D, Bier B, Rempe D, Pschadka G, Krech R, Bocker W (1998) Gains and losses of CD44 expression during breast carcinogenesis and tumour progression. Histopathology 33(2):107–116
Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF (2007) The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 356(3):217–226. doi:10.1056/NEJMoa063994
Alison MR, Lim SM, Nicholson LJ (2011) Cancer stem cells: problems for therapy? J Pathol 223(2):147–161. doi:10.1002/path.2793
Acknowledgments
We thank Professor Peter Altvoget for the generous gift of anti-CD24 (SWA11) antibody. We also thank the Ministry of Higher Education (Egypt) for funding Mohamed Ahmed and M. Aleskandarany.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2011_1865_MOESM1_ESM.tif
Supplementary Fig. 1: CD24 Nuclear expression. TMA core showing nuclear immunohistochemical expression of CD24; (A): X100, and (B): ×200. (C) and (D): Kaplan–Meier survival plot for patients’ BCSS and MFS for nuclear expression of CD24. Supplementary material 1 (EPS 2849 kb)
Rights and permissions
About this article
Cite this article
Ahmed, M.A.H., Aleskandarany, M.A., Rakha, E.A. et al. A CD44−/CD24+ phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat 133, 979–995 (2012). https://doi.org/10.1007/s10549-011-1865-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1865-8