Abstract
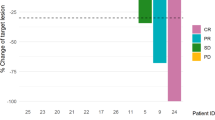
The epothilone B analogue, ixabepilone, binds to β-tubulin, is effective for taxane-refractory metastatic breast cancer (MBC), and may be given every 3 weeks or weekly. We evaluated the efficacy of weekly ixabepilone (I) plus trastuzumab (T) and carboplatin (C) as first line therapy in HER2 + MBC. Patients with HER2+ (3+ by IHC or FISH amplified) MBC received I (15 mg/m2 IV) and C (area under the curve, AUC = 2 IV) on days 1, 8, and 15 of a 28-day cycle for a maximum of 6 cycles, plus weekly T (4 mg/kg loading dose then 2 mg/kg IV) during chemotherapy then every 3 weeks (6 mg/kg IV) until disease progression. The primary objective was to determine whether the combination was associated with a response rate (RR) of at least 75%. Fifty-nine patients were treated, and 39 had HER2 overexpression confirmed in a central lab (cHER2+). For all treated patients, objective response occurred in 26 patients (44%; 95% CI 31–58%), median time to progression was 8.2 months (95% CI 6.3–9.9), and median overall survival was 34.7 months (95% CI 25.7 to [not reached]). Results were comparable for cHer2+ cancers. Grade 3–4 adverse events included neutropenia (49%), thrombocytopenia (14%), fatigue (12%), nausea (7%), diarrhea (7%), and neuropathy (7%). One patient died from treatment complications during cycle 1. Weekly ixabepilone and carboplatin plus trastuzumab have an acceptable toxicity profile, but are not likely to be associated with an RR of 75% in HER2+ MBC. Efficacy appears comparable to paclitaxel, carboplatin, and trastuzumab.


Similar content being viewed by others
References
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Burris H III, Yardley D, Jones S et al (2004) Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol 22:1621–1629
Ruiz M, Salvador J, Bayo J et al (2008) Phase-II study of weekly schedule of trastuzumab, paclitaxel, and carboplatin followed by a week off every 28 days for HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 62:1085–1090
Pegram MD, Pienkowski T, Northfelt DW et al (2004) Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst 96:759–769
Perez EA, Suman VJ, Rowland KM et al (2005) Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer 6:425–432
Robert N, Leyland-Jones B, Asmar L et al (2006) Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 24:2786–2792
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–1649
Gori S, Mosconi AM, Basurtol C et al (2002) Weekly paclitaxel in metastatic breast cancer patients: a phase II study. Tumori 88:470–473
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23:5983–5992
Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R (2001) Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol 19:4216–4223
Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH (2004) The binding mode of epothilone A on alpha, beta-tubulin by electron crystallography. Science 305:866–869
Lee FY, Borzilleri R, Fairchild CR et al (2001) BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res 7:1429–1437
Perez EA, Lerzo G, Pivot X et al (2007) Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol 25:3407–3414
Thomas E, Tabernero J, Fornier M et al (2007) Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol 25:3399–3406
Low JA, Wedam SB, Lee JJ et al (2005) Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol 23:2726–2734
Roche H, Yelle L, Cognetti F et al (2007) Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol 25:3415–3420
Denduluri N, Low JA, Lee JJ et al (2007) Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol 25:3421–3427
Awada A, Piccart MJ, Jones SF et al (2009) Phase I dose escalation study of weekly ixabepilone, an epothilone analog, in patients with advanced solid tumors who have failed standard therapy. Cancer Chemother Pharmacol 63:417–425
Plummer R, Woll P, Fyfe D et al (2008) A phase I and pharmacokinetic study of ixabepilone in combination with carboplatin in patients with advanced solid malignancies. Clin Cancer Res 14:8288–8294
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Kaplan EL, Meier P (1958) Nonparametric estimator from incomplete observations. J Am Stat Assoc 53:457–481
Rugo HS, Campone M, Amadori A et al (2009) Randomized phase II study of weekly versus every-3-week ixabepilone plus bevacizumab (ixa/bev) versus paclitaxel plus bev (pac/bev) as first-line therapy for metastatic breast cancer (MBC). J Clin Oncol 27:15s (suppl; abstr 1029)
Sparano JA (2000) Taxanes for breast cancer: an evidence-based review of randomized phase II and phase III trials. Clin Breast Cancer 1:32–40 discussion 1–2
Rivera E, Mejia JA, Arun BK et al (2008) Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast cancer. Cancer 112:1455–1461
Fromes Y, Gounon P, Veitia R, Bissery MC, Fellous A (1996) Influence of microtubule-associated proteins on the differential effects of paclitaxel and docetaxel. J Protein Chem 15:377–388
Gueritte-Voegelein F, Guenard D, Lavelle F, Le Goff MT, Mangatal L, Potier P (1991) Relationships between the structure of taxol analogues and their antimitotic activity. J Med Chem 34:992–998
Verweij J, Clavel M, Chevalier B (1994) Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol 5:495–505
Pegram MF, Forbes J, Pienkowski T et al (2007) BCIRG 007: first overall survival analysis of randomized phase III trial of trastuzumab plus docetaxel with or without carboplatin as first line therapy in HER2 amplified metastatic breast cancer (MBC). J Clin Oncol 2007; ASCO annual meeting proceedings, vol 25
Acknowledgment
This study was supported in part by grants from the Department of Health and Human Services and the National Institutes of Health (CA23318 to the ECOG statistical center, CA66636 to the ECOG data management center, CA21115 to the ECOG coordinating center, CA25224 to NCCTG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moulder, S., Li, H., Wang, M. et al. A phase II trial of trastuzumab plus weekly ixabepilone and carboplatin in patients with HER2-positive metastatic breast cancer: an Eastern Cooperative Oncology Group Trial. Breast Cancer Res Treat 119, 663–671 (2010). https://doi.org/10.1007/s10549-009-0658-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0658-9