Abstract
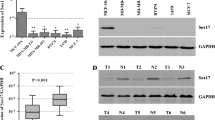
SRY-box 17 (Sox17) is a transcription factor which involved in a variety of developmental processes and can act as an antagonist of canonical Wnt/β-catenin signaling pathway. However, the relationship between Sox17 gene expression, methylation status, and β-catenin in breast cancer has not been established. Here we report that the expression level of Sox17 mRNA was dramatically decreased in five different breast cancer cell lines and 23 of 31 primary breast tumor samples, which significantly correlated with its methylation status. After treated with 5-aza-2′-deoxycytidine (5-aza-dC, a demethylation agent), the expression levels of Sox17 mRNA and protein were obviously increased. Restored expression of Sox17 by 5-aza-dC treatment decreased the expression level of β-catenin in breast cancer cell lines. Furthermore, small interfering RNA (siRNA)-mediated knockdown of Sox17 in SKBR-3 and Bacp-37 cells enhanced β-catenin expression. In 31 paired tissue samples, a significant difference between the expression level of Sox17 and β-catenin was also observed (P < 0.001). Clinically, Sox17 methylation was detected in 74.3% breast tumors (84/113) and 31.9% (36/113) paired normal tissues, respectively (P < 0.0001). Sox17 methylation was also associated with tumor stage (P = 0.028) and lymph node metastasis (P = 0.013). These findings indicate that silencing of Sox17 due to promoter hypermethylation is a frequent event and may contribute to aberrant activation of Wnt signaling in breast cancer. Sox17 may be a valuable biomarker for the study of breast cancer carcinogenesis and progression.





Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66
Herman JG, Baylin SB (2003) Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 349:2042–2054. doi:10.1056/NEJMra023075
Das PM, Singal R (2007) DNA methylation and cancer. J Clin Oncol 22:4632–4642. doi:10.1200/JCO.2004.07.151
Jones PA, Baylin SB (2007) The epigenomics of cancer. Cell 128:683–692. doi:10.1016/j.cell.2007.01.029
Szyf M, Pakneshan P, Rabbani SA (2004) DNA methylation and breast cancer. Biochem Pharmacol 68:1187–1197. doi:10.1016/j.bcp.2004.04.030
Agrawal A, Murphy RF, Agrawal DK (2007) DNA methylation in breast and colorectal cancers. Mod Pathol 20:711–721. doi:10.1038/modpathol.3800822
Kamachi Y, Uchikawa M, Kondoh H (2000) Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet 16:182–187. doi:10.1016/S0168-9525(99)01955-1
Wegner M (1999) From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 27:1409–1420. doi:10.1093/nar/27.6.1409
Kiefer JC (2007) Back to basics: Sox genes. Dev Dyn 236:2356–2366. doi:10.1002/dvdy.21218
Lefebvre V, Dumitriu B, Penzo-Méndez A et al (2007) Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol 39:2195–2214. doi:10.1016/j.biocel.2007.05.019
Dong C, Wilhelm D, Koopman P (2004) Sox genes and cancer. Cytogenet Genome Res 105:442–447. doi:10.1159/000078217
Sohn J, Natale J, Chew LJ, Belachew S et al (2006) Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. J Neurosci 26:9722–9735. doi:10.1523/JNEUROSCI.1716-06.2006
Matsui T, Kanai-Azuma M, Hara K et al (2006) Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci 119:3513–3526. doi:10.1242/jcs.03081
Park KS, Wells JM, Zorn AM et al (2006) Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol 294:192–202. doi:10.1016/j.ydbio.2006.02.038
Shimoda M, Kanai-Azuma M, Hara K et al (2007) Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J Cell Sci 120:3859–3869. doi:10.1242/jcs.007856
Kim I, Saunders TL, Morrison SJ (2007) Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130:470–483. doi:10.1016/j.cell.2007.06.011
Jang YY, Sharkis SJ (2007) Fetal to adult stem cell transition: knocking Sox17 off. Cell 130:403–404. doi:10.1016/j.cell.2007.07.027
Liu Y, Asakura M, Inoue H et al (2007) Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci USA 104:3859–3864. doi:10.1073/pnas.0609100104
Sinner D, Kordich JJ, Spence JR et al (2007) Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol 27:7802–7815. doi:10.1128/MCB.02179-06
Zhang W, Glöckner SC, Guo M et al (2008) Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer. Cancer Res 68:2764–2772. doi:10.1158/0008-5472.CAN-07-6349
Zorn AM, Barish GD, Williams BO et al (1999) Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell 4:487–498. doi:10.1016/S1097-2765(00)80200-2
Kanai-Azuma M, Kanai Y, Gad JM et al (2002) Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129:2367–2379
de Jong J, Stoop H, Gillis AJ et al (2008) Differential expression of Sox17 and SOX2 in germ cells and stem cells has biological and clinical implications. J Pathol 215:21–30. doi:10.1002/path.2332
Morin PJ, Weeraratna AT (2003) Wnt signaling in human cancer. Cancer Treat Res 115:169–187. doi:10.1007/0-306-48158-8_7
Karim R, Tse G, Putti T et al (2004) The significance of the Wnt pathway in the pathology of human cancers. Pathology 36:120–128. doi:10.1080/00313020410001671957
Brantjes H, Barker N, van Es J et al (2002) Lady justice casting the final verdict on the outcome of Wnt signalling. Biol Chem 383:255–261. doi:10.1515/BC.2002.027
Li Y, Welm B, Podsypanina K et al (2003) Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA 100:15853–15858. doi:10.1073/pnas.2136825100
Howe LR, Brown AM (2004) Wnt signaling and breast cancer. Cancer Biol Ther 3:36–41
Veeck J, Niederacher D, An H et al (2006) Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene 25:3479–3488. doi:10.1038/sj.onc.1209386
Lo PK, Mehrotra J, D’Costa A et al (2006) Epigenetic suppression of secreted frizzled related protein 1 (SFRP1) expression in human breast cancer. Cancer Biol Ther 5:281–286
Veeck J, Geisler C, Noetzel E et al (2008) Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis 29:991–998. doi:10.1093/carcin/bgn076
Ai L, Tao Q, Zhong S et al (2006) Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis 27:1341–1348. doi:10.1093/carcin/bgi379
Suzuki H, Toyota M, Carraway H et al (2008) Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer 98:1147–1156. doi:10.1038/sj.bjc.6604259
Akiyama H, Lyons JP, Mori-Akiyama Y et al (2004) Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 18:1072–1087. doi:10.1101/gad.1171104
Takash W, Cañizares J, Bonneaud N et al (2001) SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res 29:4274–4283. doi:10.1093/nar/29.21.4274
Sinner D, Rankin S, Lee M et al (2004) Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 131:3069–3080. doi:10.1242/dev.01176
Katoh M (2002) Molecular cloning and characterization of human Sox17. Int J Mol Med 9:153–157
Feltus FA, Lee EK, Costello JF et al (2004) Predicting aberrant CpG island methylation. Proc Natl Acad Sci USA 100:12253–12258. doi:10.1073/pnas.2037852100
Ushijima T (2005) Detection and interpretation of altered methylation patterns in cancer cells. Nat Rev Cancer 5:23–31. doi:10.1038/nrc1571
Kimelman D, Xu W (2006) Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25:7482–7491. doi:10.1038/sj.onc.1210055
Lin SY, Xia W, Wang JC et al (2000) Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97:4262–4266. doi:10.1073/pnas.060025397
Turashvili G, Bouchal J, Burkadze G et al (2006) Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73:213–223. doi:10.1159/000098207
Dolled-Filhart M, McCabe A, Giltnane J et al (2006) Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res 66:5487–5494. doi:10.1158/0008-5472.CAN-06-0100
Candidus S, Bischoff P, Becker KF et al (1996) No evidence for mutations in the alpha- and beta-catenin genes in human gastric and breast carcinomas. Cancer Res 56:49–52
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116:2627–2634. doi:10.1242/jcs.00623
Acknowledgments
The authors thank the studied patients for their willingness to cooperate with our study. This research was supported in part by the grants from the National Basic Research Program of China (2006CB910501, 2004CB518605), National Natural Science Foundation of China (30371580, 30572109); Chinese National Natural Science Fund for Distinguished Young Scholars (30625019); Shanghai Science and Technology Committee (03J14019, 06DJ14004, 06DZ19504).
Author information
Authors and Affiliations
Corresponding authors
Additional information
De-Yuan Fu and Zhi-Min Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fu, DY., Wang, ZM., Li-Chen et al. Sox17, the canonical Wnt antagonist, is epigenetically inactivated by promoter methylation in human breast cancer. Breast Cancer Res Treat 119, 601–612 (2010). https://doi.org/10.1007/s10549-009-0339-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0339-8