Abstract
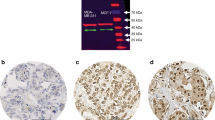
The farnesoid X receptor (FXR, NR1H4), a member of the nuclear receptor superfamily of ligand-dependent transcription factors, is normally produced in the liver and the gastrointestinal tract, where it acts as a bile acid sensor. It has been recently detected in breast cancer cell lines and tissue specimens. The expression of FXR was scored (0–8) by immunohistochemistry on 204 breast cancer samples and correlated with established cancer biomarkers. Moreover, the effect of the FXR activator chenodeoxycholic acid (CDCA) was determined on cell proliferation and estrogen receptor regulation/activation in breast cancer cell lines. FXR was detected in 82.4% of samples with a high median expression score of 5. FXR expression significantly correlated with estrogen receptor (ER) expression (P = 0.009) and luminal-like markers. In ER-positive tumors, FXR expression was significantly correlated with the proliferation marker Ki-67 (P < 0.001) and the nodal status (P = 0.028), but only so in postmenopausal women, suggesting that lack of estrogens may disclose the association between FXR and cell proliferation. In vitro experiments confirmed clinical data since CDCA stimulated the proliferation of ER-positive cells only in steroid-free medium, a stimulation inhibited upon siRNA-silencing of FXR expression as well as ER blockade by antiestrogens. Moreover, co-immunoprecipitation experiments revealed that CDCA activated-FXR interacted with ER. These results suggest that ER-positive breast tumors could be stimulated to proliferate via a crosstalk between FXR and ER, particularly in a state of estrogen deprivation (menopause, aromatase inhibitors).





Similar content being viewed by others
References
Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW et al (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81:687–693
Wang H, Chen J, Hollister K, Sowers LC, Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD et al (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368
Modica S, Moschetta A (2006) Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett 580:5492–5499
Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y (2006) FXR, a multipurpose nuclear receptor. Trends Biochem Sci 31:572–580
Edwards PA, Kast HR, Anisfeld AM (2002) BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res 43:2–12
Kalaany NY, Mangelsdorf DJ (2006) LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol 68:159–191
Claudel T, Sturm E, Kuipers F, Staels B (2004) The farnesoid X receptor: a novel drug target? Expert Opin Investig Drugs 13:1135–1148
Westin S, Heyman RA, Martin R (2005) FXR, a therapeutic target for bile acid and lipid disorders. Mini Rev Med Chem 5:719–727
Bishop-Bailey D, Walsh DT, Warner TD (2004) Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA 101:3668–3673
He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S et al (2006) Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res 98:192–199
Silva J, Dasgupta S, Wang G, Krishnamurthy K, Ritter E, Bieberich E (2006) Lipids isolated from bone induce the migration of human breast cancer cells. J Lipid Res 47:724–733
Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D (2006) The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res 66:10120–10126
Journe F, Laurent G, Chaboteaux C, Nonclercq D, Durbecq V, Larsimont D, Body JJ (2008) Farnesol, a mevalonate pathway intermediate, stimulates MCF-7 breast cancer cell growth through farnesoid-X-receptor-mediated estrogen receptor activation. Breast Cancer Res Treat 107:49–61
Costarelli V, Sanders TA (2002) Plasma deoxycholic acid concentration is elevated in postmenopausal women with newly diagnosed breast cancer. Eur J Clin Nutr 56:925–927
Raju U, Levitz M, Javitt NB (1990) Bile acids in human breast cyst fluid: the identification of lithocholic acid. J Clin Endocrinol Metab 70:1030–1034
Javitt NB, Budai K, Miller DG, Cahan AC, Raju U, Levitz M (1994) Breast-gut connection: origin of chenodeoxycholic acid in breast cyst fluid. Lancet 343:633–635
Costarelli V, Sanders TA (2002) Plasma bile acids and risk of breast cancer. IARC Sci Publ 156:305–306
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ (2005) Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol 16:1723–1739
Prall OW, Sarcevic B, Musgrove EA, Watts CK, Sutherland RL (1997) Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem 272:10882–10894
Foster JS, Henley DC, Bukovsky A, Seth P, Wimalasena J (2001) Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function. Mol Cell Biol 21:794–810
Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9:1149–1163
De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, Bentzen CL, Niesor EJ, Dufour JF (2004) The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci 49:982–989
De Gottardi A, Dumonceau JM, Bruttin F, Vonlaufen A, Morard I, Spahr L, Rubbia-Brandt L, Frossard JL, Dinjens WN, Rabinovitch PS et al (2006) Expression of the bile acid receptor FXR in Barrett’s esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol Cancer 5:48
Shishodia S, Aggarwal BB (2004) Guggulsterone inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J Biol Chem 279:47148–47158
Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM et al (1997) High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA 94:6380–6385
Sweeney KJ, Swarbrick A, Sutherland RL, Musgrove EA (1998) Lack of relationship between CDK activity and G1 cyclin expression in breast cancer cells. Oncogene 16:2865–2878
Hui R, Cornish AL, McClelland RA, Robertson JF, Blamey RW, Musgrove EA, Nicholson RI, Sutherland RL (1996) Cyclin D1 and estrogen receptor messenger RNA levels are positively correlated in primary breast cancer. Clin Cancer Res 2:923–928
Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L et al (1997) Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med 3:227–230
Sherr CJ (1996) Cancer cell cycles. Science 274:1672–1677
Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13:1501–1512
Mateyak MK, Obaya AJ, Sedivy JM (1999) c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol 19:4672–4683
Brodie AM, Njar VC (2000) Aromatase inhibitors and their application in breast cancer treatment*. Steroids 65:171–179
Blankenstein MA, van de Ven J, Maitimu-Smeele I, Donker GH, de Jong PC, Daroszewski J, Szymczak J, Milewicz A, Thijssen JH (1999) Intratumoral levels of estrogens in breast cancer. J Steroid Biochem Mol Biol 69:293–297
Nagengast FM, Grubben MJ, van Munster IP (1995) Role of bile acids in colorectal carcinogenesis. Eur J Cancer 31A:1067–1070
Lewis SJ, Heaton KW (1999) The metabolic consequences of slow colonic transit. Am J Gastroenterol 94:2010–2016
Carmichael AR (2006) Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG 113:1160–1166
Halmy L, Feher T, Steczek K, Farkas A (1986) High serum bile acid level in obesity: its decrease during and after total fasting. Acta Med Hung 43:55–58
Cho E, Spiegelman D, Hunter DJ, Chen WY, Stampfer MJ, Colditz GA, Willett WC (2003) Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst 95:1079–1085
Costarelli V, Sanders TA (2001) Acute effects of dietary fat composition on postprandial plasma bile acid and cholecystokinin concentrations in healthy premenopausal women. Br J Nutr 86:471–477
Harlan LC, Coates RJ, Block G, Greenberg RS, Ershow A, Forman M, Austin DF, Chen V, Heymsfield SB (1993) Estrogen receptor status and dietary intakes in breast cancer patients. Epidemiology 4:25–31
Kushi LH, Potter JD, Bostick RM, Drinkard CR, Sellers TA, Gapstur SM, Cerhan JR, Folsom AR (1995) Dietary fat and risk of breast cancer according to hormone receptor status. Cancer Epidemiol Biomarkers Prev 4:11–19
Jain M, Miller AB (1997) Tumor characteristics and survival of breast cancer patients in relation to premorbid diet and body size. Breast Cancer Res Treat 42:43–55
Journe F, Durbecq V, Chaboteaux C, Rouas G, IdBoufker H, Laurent G, Larsimont D, Body J (2008) Farnesoid X receptor in primary breast carcinoma: a new marker to predict bone metastasis? Bone 42(Suppl 1):S97 (abstract 175)
Acknowledgements
This study received financial support from the “Fondation Medic”, from the “Fondation contre le Cancer”, from the Belgian Fund for Medical Scientific Research (grants no. 3.4512.03 and 3.4501.08), from the “Fondation Lambeau-Marteaux”, and from “Les Amis de l’Institut Bordet”. Virginie Durbecq, Christos Sotiriou and Guy Laurent are respectively Scientific Research Worker, Research Associate and Senior Research Associate of the National Fund for Scientific Research (Belgium).
Author information
Authors and Affiliations
Corresponding author
Additional information
Fabrice Journe and Virginie Durbecq had contributed equally to this work and should be considered as joint first authors.
Rights and permissions
About this article
Cite this article
Journe, F., Durbecq, V., Chaboteaux, C. et al. Association between farnesoid X receptor expression and cell proliferation in estrogen receptor-positive luminal-like breast cancer from postmenopausal patients. Breast Cancer Res Treat 115, 523–535 (2009). https://doi.org/10.1007/s10549-008-0094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0094-2