Abstract
Current research focuses on the soluble and high-level expression of biologically active recombinant human IL-29 protein in Escherichia coli. The codon-optimized IL-29 gene was cloned into the Champion™ pET SUMO expression system downstream of the SUMO tag under the influence of the T7 lac promoter. The expression of SUMO-fused IL-29 protein was compared in E. coli Rosetta 2(DE3), Rosetta 2(DE3) pLysS, and Rosetta-gami 2(DE3). The release of the SUMO fusion partner resulted in approximately 98 mg of native rhIL-29 protein with a purity of 99% from 1 l of fermentation culture. Purified rhIL-29 was found to be biologically active, as evaluated by its anti-proliferation assay. It was found that Champion™ pET SUMO expression system can be used to obtained high yield of biologically active soluble recombinant human protein compared to other expression vector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human IL-29 is a pleiotropic cytokine produced primarily by monocyte-derived dendritic cells, epithelial cells, and peripheral blood mononuclear cells (PBMCs) in response to viral infections and lipopolysaccharide (LPS) stimulation (Kotenko et al. 2003; Sheppard et al. 2003). IL-29 is the most abundant type III interferon in human serum, while it exists as a pseudogene in mice and is the only IFN-λ that exhibits N-linked glycosylation (Donnelly and Kotenko 2010). IL-29 mainly targets hepatocytes and epithelial tissues (Lazear et al. 2015b) and protects endothelial cells of the spleen, kidney, and central nervous system, as observed with type I interferons (Sommereyns et al. 2008). It generates a milder but prolonged response with equal efficacy as IFN-α (Muir et al. 2014). Type III interferon family member genes were clustered on chromosome 19 (q13.13). Although their genomic structure is similar to that of the IL-10 family, their protein signaling resembles that of type I interferons rather than that of IL-10. Therefore, these proteins have been independently described as IFN-λ1 (IL-29), IFN-λ2 (IL-28A), IFN-λ3 (IL-28B), and IFN-λ4 (Kotenko et al. 2003; Donnelly and Kotenko 2010; Prokunina-Olsson et al. 2013). Not surprisingly, IFN I-like interferon-stimulating genes (ISGs) have been reported to be triggered by type III IFNs that inhibit the infection of numerous types of viruses in a variety of cells (Ank et al. 2006).
In vitro cellular systems have demonstrated that IL-29 is a potential inhibitor of replication of hepatitis B virus (HBV), Hepatitis C virus (HCV) (Robek et al. 2005), human immunodeficiency virus type 1 (HIV-1) (Hou et al. 2009), West Nile virus (WNV) (Lazear et al. 2015a), and dengue virus (Palma-Ocampo et al. 2015). IFN-λ has been reported to be potent against coronaviruses, including MERS-CoV and SARS-CoV1 (Mahlakoiv et al. 2012; Hamming et al. 2013). IL-29 has recently been proposed as a potential in treating COVID-19 (SARS-CoV2) (Prokunina-Olsson et al. 2020). Clinical trials evaluating the tolerability, safety, and effectiveness of PEGylated IL-29 for SARS-CoV2 are under way (ClinicalTrials.gov Identifier: NCT04343976).
Studies have shown that IFN-λ can exert direct biological effects on tumor cell growth and functions, such as reducing tumorigenicity, inducing cell cycle arrest, and causing apoptosis through multiple mechanisms (Lasfar et al. 2011). Several preclinical trials have reported IFN-λ-mediated antitumor effects on human and murine cancer cells, such as colon cancer (Hui et al. 2011), esophageal carcinoma (Li et al. 2010), lung cancer (Tezuka et al. 2012), and neuroendocrine tumors (Zitzmann et al. 2006).
Despite its significance, the production of this therapeutically important protein remains a challenging task. Researchers have expressed IL-29 in Escherichia coli in the form of inclusion bodies as they are often misfolded, which require further downstream processing to obtain active recombinant proteins (Li & He 2006; Li & Huang 2007; Wang et al. 2011). However, all these processes reduce the final protein yields. Moreover, proteins expressed in the form of inclusion bodies are partially misfolded, which increases the risk of adverse immune reactions.
Therefore, therapeutic proteins must be produced in a soluble, biologically active form to avoid adverse effects and simplify downstream processes. Many methods have been developed to improve soluble protein production in E. coli (Cabrita et al. 2006; Rabhi-Essafi et al. 2007; Burgess-Brown et al. 2008). The fusion approach is a common strategy for enhancing efficient expression and simplifying purification. Widely used fusion tags include GST (Rabhi-Essafi et al. 2007), Nus A (De Marco et al. 2004), MBP (Kapust & Waugh 1999) and SUMO (Butt et al. 2005). SUMO fusion technology enables high-level expression through proper folding of the target protein due to its chaperone-like effect (Kong & Guo 2011) and detergent-like effect on insoluble target proteins (Malakhov et al. 2004). This fusion technology not only increases the stability of the construct, but also improves the solubility of the desired protein; hence, it is considered a useful method for the expression of difficult-to-express proteins.
In this study, we focused on soluble and high-level expression of biologically active IL-29 protein in E. coli using the Champion™ pET SUMO expression system. The purification of the pET SUMO-IL-29 protein possessing a His-tag at the N-terminus, proteolytic cleavage to obtain the native protein by the release of SUMO, and the anti-proliferative activity of rhIL-29 protein were determined.
Materials and methods
Materials
The Champion™ pET SUMO expression vector (K30001) and PureLink™ Quick Gel Extraction Kit (K210012) were purchased from Invitrogen. DreamTaq Green PCR Master Mix (K1082), T4 DNA ligase (5U/µl), Rapid DNA Ligation Kit, GeneJET Plasmid Miniprep Kit, isopropyl β-D-1-thiogalactopyranoside (IPTG), and PageRuler™ Unstained Protein ladder were obtained from Thermo Scientific™. Rosetta 2(DE3), Rosetta 2(DE3) pLysS, and Rosetta-gami™ 2(DE3) cells were purchased from Novagen, and HepG2, HCT-116, and MCF-7 cell lines for the biological assay were purchased from ATCC, USA. Terrific broth and tetracycline hydrochloride were purchased from Fisher bioreagents™, USA. Streptomycin sulfate, chloramphenicol, and a purification column (Ni-Sepharose column) were purchased from Sigma. The Ni-Sepharose™ 6 Fast Flow resin was purchased from GE Healthcare Life Sciences. Kanamycin monosulfate was obtained from ICN Biomedicals, Inc. IL28/29 (H-1) sc-365834 mouse monoclonal IgG2a antibody and goat anti-mouse IgG-AP-conjugated antibody were purchased from Santa Cruz Biotechnology. The Rainbow™ Full-range Molecular Marker (Mr 12,000–225,000) was purchased from GE Healthcare Life Sciences. DMEM medium, fetal bovine serum, penicillin–streptomycin and trypsin were purchased from Gibco.
Methods
Construction of pET SUMO-IL-29 clone
The codon-optimized IL-29 gene (Accession #OP866269) was amplified using forward and reverse primers 5′-GGTCCGGTGCCGACGAGT-3′ and 5′-CTAGTCTAGATTAGGTAGATTCCGGGTG-3′, respectively. Both primers were designed using the ExPASy Bioinformatics tool and synthesized by Eurofins Genomics. Using DreamTaq PCR master mix (Thermo Scientific™), PCR amplification of IL-29 was carried out that consisted of 25 cycles with a denaturation step at 94 °C for 2 min, followed by 94 °C for 30 s, annealing at 58 °C for 30 s, and 72 °C for 30 s, and a final extension step at 72 °C for 7 min. The amplified product was visualized on a 1.5% agarose gel using Ethidium Bromide as the staining agent. The amplified DNA band was excised and eluted using the PureLink™ Quick Gel Extraction Kit (Invitrogen). Purified DNA was ligated into the pET SUMO expression vector (1:1) using T4 DNA ligase (5U/µl). The ligation mixture was then incubated overnight at 22 °C, and the ligated product was transformed into chemically competent E. coli TOP10F’ cells. Confirmation of positive clones was based on 12.5 µg/ml tetracycline and 50 µg/ml kanamycin. Following the selection of a single colony, DNA was isolated using the GeneJET Plasmid Miniprep Kit and eluted in Milli-Q water to check for the correct insertion of IL-29 into the pET SUMO vector by sequencing using T7 universal primer (Eurofins Genomics).
Expression of recombinant pET SUMO-IL-29 protein
Escherichia coli Rosetta 2(DE3), Rosetta 2(DE3) pLysS, and Rosetta-gami 2(DE3) cells were made competent using CaCl2 in heat shock transformation method to express pET SUMO-IL-29. Each transformation culture was transferred directly to 10 ml LB medium supplemented with 10 µl of 50 µg/ml kanamycin and 34.5 µg/ml chloramphenicol for Rosetta 2(DE3) and Rosetta 2(DE3) pLysS, and 34.5 µg/ml chloramphenicol, 50 µg/ml streptomycin, 12.5 µg/ml tetracycline, and 50 µg/ml kanamycin for Rosetta-gami 2(DE3). Primary cultures were grown overnight at 210 rpm and 37 °C in a shaker incubator. They were diluted ten times by transferring them to 10 ml LB media containing 50 µg/ml kanamycin and grown at 37 °C with shaking at 210 rpm. Bacterial cultures were allowed to grow until the absorbance at 600 nm reached 0.4–0.6 (mid-log phase). 0.1 M isopropyl β-D-1-thiogalactopyranoside (IPTG) was added at a final concentration of 1 mM to induce culture in the expression of IL-29. The cells were collected every 2 h to determine the optimum time required for maximum protein yield. Protein samples were prepared using reducing 2X Laemmli Buffer and was analyzed using 12% SDS-PAGE after staining with the Coomassie stain. Protein quantification was performed using ImageJ software to compare protein concentrations in all expression hosts.
Large scale expression of pET SUMO-IL-29
Rosetta-gami 2(DE3) cells were transformed using the heat shock method for large-scale expression of IL-29. The transformation mixture was inoculated in 200 ml LB broth supplemented with 34.5 µg/ml chloramphenicol, 50 µg/ml streptomycin, 12.5 µg/ml tetracycline, and 50 µg/ml kanamycin and grown overnight at 210 rpm and 37 °C. The secondary culture was prepared by diluting the primary culture into 2 l baffled shake flasks containing 450 ml of Terrific Broth in each medium supplemented with 50 µg/ml kanamycin. The culture was grown for 6–8 h at 37 °C and 210 rpm. When the culture reached the mid-log phase (OD600 = 0.8–1), IPTG (0.1 M) was added at a final concentration of 1 mM. After 4–5 h of induction, bacterial cells were harvested at 5000×g for 10 min at 4 °C.
Lysis of biomass
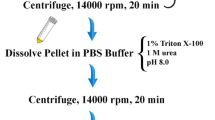
Cell lysis was performed using a French press cell disruption system (Constant Systems Limited) at 1.5 Bar pressure by adding 100 ml equilibration buffer (20 mM sodium phosphate, 0.5 M NaCl, pH7.4) to the cell pellet. To prevent degradation, 100 mM phenylmethylsulfonyl (PMSF) was added to a final concentration of 0.1 mM before lysis. The lysate was then centrifuged at 7000×g for 20 min at 4 °C. Lysate (soluble protein), crude sample (total protein), and cell pellet obtained after lysis (insoluble protein) were analyzed using 12% SDS-PAGE. After de-staining, a high-resolution image of the gel was taken. It was then subjected to densitometry analysis using ImageJ software (NIH, USA). The peak area for each sample (lysate, total crude protein, and cell pellet obtained after lysis) was calculated to estimate the protein concentration in each sample. The peak areas were plotted to compare the protein concentrations.
Purification of pSUMO-IL-29 fusion protein
The supernatant containing soluble IL-29 was purified using ready to use HisTrap™ HP, 5 ml Affinity column of immobilized metal affinity chromatography (IMAC). The SUMO fused protein was purified using Ni-Sepharose column packed with Ni-Sepharose™ 6 Fast Flow resin (GE Healthcare Life Sciences™) on ÄKTA system (GE Healthcare, USA). For equilibration, 20 column volumes of PBS (pH 7.4) were passed through the column at a flow rate of 5 ml/min. The protein sample was loaded onto the column at 2.5 ml/min flow rate. Then, five column volumes of equilibration buffer at 5 ml/min flow rate were passed to wash the column. To avoid non-specific protein binding, the column was washed with five column volume of buffer (PBS, pH 7.4, 20 mM imidazole) at 5 ml/min. The pSUMO-IL-29 protein was eluted from the column using an elution buffer (PBS, pH 7.4, 500 mM imidazole) at 5 ml/min till the protein was eluted. The collected protein fractions were analyzed by western blotting and quantified by UV/vis spectrometry at 280 nm. Western blotting was performed following a protocol of Bjerrum et al., 1986. After transferring the gel to the blot, it was blocked with 5% skim milk overnight at 4 °C. The blot was then incubated with monoclonal anti-IL-29 antibody and later with goat anti-mouse IgG-AP at room temperature. It was then developed using NBT/BCIP substrate to visualize IL-29 bands.
SUMO digestion of purified pSUMO-IL-29 protein
To decrease the salt concentration, dialysis was performed overnight in the presence of 20 mM PBS at 4 °C using minidialysis kit from Cytiva. For the digestion reaction, pSUMO-IL-29 protein (1 mg/ml) was incubated with 20 U SUMO protease enzyme for 4 h at 4 °C. The samples were collected after 2, 3, and 4 h. Digestion was analyzed using 12% SDS-PAGE. 20 ml of SimplyBlue™ Safe Stain (Thermo Scientific™) were used to stain the gel at room temperature for 1 h with gentle shaking. The gel was then de-stained with distilled water until bands appeared.
IMAC purification of native IL-29
Single step purification of native IL-29 was done by affinity chromatography on ÄKTA system (GE Healthcare, USA). SUMO digested sample was loaded on Ni-Sepharose column (Sigma) packed with Ni-Sepharose™ 6 Fast Flow resin (GE Healthcare Life Sciences™) on ÄKTA system. Native IL-29 protein was obtained through flow-through. Furthermore, the uncleaved fusion protein and SUMO tag were eluted using 800 mM imidazole. The protein fraction was quantified using the Bradford assay and analyzed using 12% SDS-PAGE. Bradford assay was performed by taking the absorbance of purified IL-29 and the known standards of 1–5 mg/ml BSA solution and comparing the concentration of IL-29 with the known concentration of BSA. The fraction containing native IL-29 protein was buffer-exchanged using PD-10 desalting column (GE Healthcare) pre-packed with Sephadex G-25 and subjected to further protein characterization.
Protein characterization via HPLC analysis
RP-HPLC was performed using a C18 column (4.6 × 250 mm2) and a Shimadzu liquid chromatography system using mobile phases A (300 ml acetonitrile, 1 ml trifluoroacetic acid, and 700 ml water for chromatography) and mobile phase B (800 ml acetonitrile, 1 ml trifluoroacetic acid, and 200 ml water for chromatography) at a flow rate of 1 ml/min. The sample and reference standard (IL-29 NIBSC) and 50 µl (1 mg/ml) were injected and eluted by gradient elution: 28% B, 0 min; 33% B, 5 min; 37% B, 15 min; 43% B, 10 min; 60% B, 10 min; 60% B, 2 min; 28% B, 50 min; 28% B, 60 min.
Cell viability assay
The purified recombinant IL-29 was tested for its antiproliferation activity against hepatocellular carcinoma (HepG2), colorectal cancer (HCT-116), and breast cancer cell line (MCF-7) (ATCC, USA) and compared with commercial IFNα2a (NIBSC) using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay kit according to the manufacturer’s instructions. HCT-116, HepG2, and MCF-7 cells were plated in a 96-well tissue culture plate (5 × 104 cells/well) and cultured in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin for 24 h at 37 °C in 5% CO2. The next day, the medium was refreshed with new DMEM medium, and then cells were treated with the sample and standard diluted in 1X PBS at a final concentration of 100 and 200 ng/ml with two-fold dilution in the plate. After 24 h of exposure, the medium was discarded, and the cells were incubated with 100 μl/well of 5 mg/ml MTT solution (Merck) for 1 h at 37 °C in 5% CO2. Then, 100 μl/well of DMSO was added to dissolve the crystals. The optical density was measured at 570 nm. The percentage of cytotoxicity compared to that of untreated cells as a control was determined. Experiments were performed in triplicate to evaluate the results.
Results
Construction of pET SUMO-IL-29 expression vector
The codon-optimized IL-29 gene was used in this study which was ligated in Champion™ pET SUMO expression system. This expression vector utilizes a small ubiquitin-like modifier (SUMO) that facilitates the purification and production of native proteins without the addition of any amino acids, followed by proteolytic cleavage (Müller et al. 2001). DNA sequencing followed by translation confirmed the insertion of IL-29 into the pET SUMO expression vector with the correct orientation (Fig. 1).
Expression analysis of SUMO fused IL-29
To express recombinant pET SUMO IL-29, it was transformed into Rosetta 2(DE3), Rosetta 2(DE3) pLysS, and Rosetta-gami 2(DE3) cells, and expression was optimized by varying the induction time (Fig. 2A–C). SUMO has a molecular weight of 11.5 kDa but it can migrate within a range of 15–20 kDa (or ~ 17 kDa) on SDS-PAGE (Marblestone et al. 2006). The mass of pSUMO-IL-29 was ~ 37 kDa (Fig. 2D) as was observed in 1 mM IPTG induced Rosetta-gami 2(DE3) transformed cells compared to uninduced and untransformed cells. SDS-PAGE analysis of the crude sample, lysate, and cell pellet obtained after biomass lysis was performed, as shown in Fig. 2E.
A–C Expression analysis of SUMO fused IL-29 in E. coli Rosetta 2(DE3), Rosetta 2(DE3) pLysS, and Rosetta-gami 2(DE3). Protein samples from 1 uninduced and 7 induced (2, 4, 6, 8, 10, 12, and 24 h) cells were loaded along with Thermo Scientific™ PageRuler™ Unstained Protein Ladder in 12% SDS-PAGE gel. D Expression analysis of SUMO fused IL-29 in E. coli Rosetta-gami 2(DE3). Lane L: Thermo Scientific™ PageRuler™ Unstained Protein Ladder, Lane 1: Rosetta-gami 2(DE3) cells, Lane 2: Protein from uninduced cells, Lane 3: Proteins from 1 mM IPTG induced cells expressing pSUMO-IL-29 at 37 °C for 4 h. E SDS-PAGE analysis of crude sample, lysate, and cell pellet after lysis. Lane L: Thermo Scientific™ PageRuler.™ Unstained Protein Ladder, Lane 1: Lysate, Lane 2: Crude sample, Lane 3: Lysis pellet. F PageRuler Unstained Protein Ladder (Cat # 26,614)
Purification of SUMO fused IL-29 protein
The native protein was purified by immobilized metal affinity chromatography (IMAC) because the SUMO fusion protein possesses a His-tag at the N-terminus. Western blot analysis (Fig. 3) showed successful purification of pET SUMO-IL-29 in the eluted fraction with no loss of protein in flow-through using IL28/29 (H-1) sc-365834 mouse monoclonal IgG2a antibody and goat anti-mouse IgG-AP-conjugated antibody (Santa Cruz Biotechnology).
Proteolytic cleavage and production of native IL-29
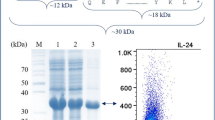
Purified pET-SUMO-IL-29 (1 mg/ml) was subjected to proteolytic cleavage using 20U of SUMO protease, resulting in ~ 98% digestion of the fusion protein. The digestion reactions of samples collected after 2, 3, and 4 h were analyzed by 12% SDS-PAGE, which showed two distinctive bands corresponding to ~ 20 kDa IL-29 and ~ 17 kDa SUMO (Fig. 4).
To obtain the soluble IL-29 protein, the SUMO-digested sample was subjected to IMAC purification. The flow-through contained the native IL-29 protein, which was characterized using 12% SDS-PAGE. SDS-PAGE analysis demonstrated that the purified IL-29 protein had a single band of 20 kDa (Fig. 5). The purity of the native IL-29 protein was 99%, as determined by SDS-PAGE (Table 1). The yield of recombinant IL-29 protein was 98 mg from 1 l of bacterial culture, as determined by the Bradford assay.
Anti-proliferation activity of purified rhIL-29
The antiproliferative activity of IL-29 was compared with that of commercially available IFNα2A in HepG2, HCT-116, and MCF-7 cell lines. Compared with IFNα2a, 200 ng/ml rhIL-29 had the highest inhibition percentage of nearly 50% (Fig. 6).
Discussion
The use of recombinant proteins has increased with the advent of various expression hosts that facilitate their soluble, biologically active, and large-scale production. Although mammalian expression systems possess many advantages (Wurm 2004), the bacterial expression system is considered the most efficient host compared to other organisms for the production of protein-based pharmaceuticals because of its various attributes, including high expression yield, cost-effectiveness, rapid protein expression, and proficient scale-up. E. coli is considered the most promising bacterial strain for recombinant protein production; however, it lacks machinery for post-translational modifications that can lead to inefficient protein folding and aggregation (in the form of inclusion bodies) (Stewart et al. 1998; Bessette et al. 1999). Aggregation can affect the biological activity and stability of therapeutic proteins and is considered a risk factor for increased immunogenicity. The major challenges in the production of therapeutic proteins from inclusion bodies are isolation, washing, solubilization, and refolding procedures (Clark 2001; Middelberg 2002). Much effort is required for the development of a robust procedure for producing industrial-grade proteins that are stable, properly folded, and free from aggregation.
The co-expression of target proteins with soluble fusion partners for heterologous protein production in E. coli can lead to enhanced expression and efficient purification. Exploiting its chaperoning properties, the SUMO tag allows high-level expression through proper folding of the target protein (Malakhov et al. 2004; Butt et al. 2005). A high soluble protein expression level can be attained using this fusion system, yielding approximately 30% of the total soluble protein fraction in E. coli (Wang et al. 2010). The SUMO protease recognizes the SUMO fusion partner, facilitating precise proteolytic cleavage, thus resulting in the production of native proteins. pET SUMO has been widely used to produce many therapeutically important proteins, including human interferon alpha-2a (Bis et al. 2014), BMP-14 (Li et al. 2011), human interleukin-11 (Nguyen et al. 2018), and FGF-21 (Wang et al. 2010).
In this study, IL-29 was cloned into the Champion™ pET SUMO expression system (Invitrogen). This efficient expression vector contains a 6X-his tag at the N-terminus of SUMO, which facilitates the effective purification of SUMO-fused IL-29 by immobilized metal affinity chromatography. The pET SUMO-IL-29 construct was transformed into Rosetta 2(DE3), Rosetta 2(DE3) pLysS, and Rosetta-gami 2(DE3) cells which showed that the highest expression of SUMO-IL-29 was observed in Rosetta-gami 2(DE3) that is also reported by Ahmed et al. (2021) when they compared BL21 (DE3), Rosetta 2 (DE3), BL21 (DE3) pLysS, and Rosetta-gami 2 (DE3) for soluble expression of rhIL-15 (Ahmed et al. 2021).
The transformant was fermented in 2L shake-flask with 0.1 mM IPTG in TB medium for large scale expression of rhIL-29. When the cells were harvested and lysed, 12% SDS-PAGE showed the soluble expression of recombinant pET SUMO-IL-29 as shown in Fig. 2E which was also observed by Kong & Guo (2011) and Marblestone et al. (2006) when they studies protein expression using SUMO fusion tag for increased solubility (Marblestone et al. 2006; Kong & Guo 2011). SUMO-fused IL-29 protein was purified from the soluble fraction using Ni-affinity chromatography which yielded 70% of 98% pure SUMO-IL-29. 5 ml of purified SUMO-IL-29 was subjected to digestion with the help of SUMO protease for the cleavage of fused SUMO. The cleaved IL-29 was further purified by IMAC chromatography with step yield of 62% and 99% purity. The yield in current study was higher (98 mg/l) in comparison to other studies who have reported as 60 mg/l (Li & He 2006), 65 mg/l (Xie et al. 2007), and 70 mg/l (Shaldzhyan et al. 2021). The antiproliferation activity of rhIL-29 in HepG2, HCT-116, and MCF-7 cell lines was consistent with previous reports (Fujie et al. 2011; Balabanov et al. 2019; Akram et al. 2023; Rahman et al. 2023), indicating that our research is successful in producing functional IL-29.
This is the first study on IL-29 expression in the soluble form of E. coli using SUMO fusion technology. This showed that the pET SUMO expression system is a promising strategy for the production of therapeutic-grade proteins with improved solubility, high expression yield, and convenient purification.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed N, Afroze B, Abbas R, Khan MA, Akram M, Tahir S, Bakht S, Munir A, Shahid AA (2021) Method for efficient soluble expression and purification of recombinant human interleukin-15. Protein Expr Purif 177:105746. https://doi.org/10.1016/j.pep.2020.105746
Akram M, Khan MA, Ahmed N, Bhatti R, Pervaiz R, Malik K, Tahir S, Abbas R, Ashraf F, Ali Q (2023) Cloning and expression of an anti-cancerous cytokine: human IL-29 gene in Chlamydomonas reinhardtii. AMB Express 13(1):23. https://doi.org/10.1186/s13568-023-01530-1
Ank N, West H, Paludan SR (2006) IFN-λ: novel antiviral cytokines. J Interferon Cytokine Res 26(6):373–379
Balabanov D, Zhao L, Zhu Z, Hunzeker ZE, Tonner HM, Ding VA, Wakefield MR, Bai Q, Fang Y (2019) IL-29 exhibits anti-tumor effect on Pan-48 pancreatic cancer cells by up-regulation of P21 and Bax. Anticancer Res 39(7):3493–3498
Bessette PH, Åslund F, Beckwith J, Georgiou G (1999) Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci 96(24):13703–13708
Bis RL, Stauffer TM, Singh SM, Lavoie TB, Mallela KM (2014) High yield soluble bacterial expression and streamlined purification of recombinant human interferon α-2a. Protein Expr Purif 99:138–146
Burgess-Brown NA, Sharma S, Sobott F, Loenarz C, Oppermann U, Gileadi O (2008) Codon optimization can improve expression of human genes in Escherichia coli: a multi-gene study. Protein Expr Purif 59(1):94–102
Butt TR, Edavettal SC, Hall JP, Mattern MR (2005) SUMO fusion technology for difficult-to-express proteins. Protein Expr Purif 43(1):1–9
Cabrita LD, Dai W, Bottomley SP (2006) A family of E. coli expression vectors for laboratory scale and high throughput soluble protein production. BMC Biotechnol 6(1):1–8
Clark EDB (2001) Protein refolding for industrial processes. Curr Opin Biotechnol 12(2):202–207
De Marco V, Stier G, Blandin S, De Marco A (2004) The solubility and stability of recombinant proteins are increased by their fusion to NusA. Biochem Biophys Res Commun 322(3):766–771
Donnelly RP, Kotenko SV (2010) Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 30(8):555–564. https://doi.org/10.1089/jir.2010.0078
Fujie H, Tanaka T, Tagawa M, Kaijun N, Watanabe M, Suzuki T, Nakayama K, Numasaki M (2011) Antitumor activity of type III interferon alone or in combination with type I interferon against human non-small cell lung cancer. Cancer Sci 102(11):1977–1990. https://doi.org/10.1111/j.1349-7006.2011.02079.x
Hamming OJ, Terczyńska-Dyla E, Vieyres G, Dijkman R, Jørgensen SE, Akhtar H, Siupka P, Pietschmann T, Thiel V, Hartmann R (2013) Interferon lambda 4 signals via the IFNλ receptor to regulate antiviral activity against HCV and coronaviruses. EMBO J 32(23):3055–3065
Hou W, Wang X, Ye L, Zhou L, Yang Z-Q, Riedel E, Ho W-Z (2009) Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol 83(8):3834–3842
Hui X, Chen H, Zhang S, Ma X, Wang X, Huang B (2011) Antitumor activities of recombinant human interferon (IFN)-λ1 in vitro and in xenograft models in vivo for colon cancer. Cancer Let 311(2):141–151
Kapust RB, Waugh DS (1999) Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 8(8):1668–1674
Kong B, Guo GL (2011) Enhanced in vitro refolding of fibroblast growth factor 15 with the assistance of SUMO fusion partner. PLoS ONE 6(5):e20307
Kotenko S, Gallagher G, Baurin V, Antes A, Shen M, Shah N, Langer J, Sheikh F, Dickensheets H, Donnelly R (2003) IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nature Immunol 4:69–77. https://doi.org/10.1038/ni875
Lasfar A, Abushahba W, Balan M, Cohen-Solal KA (2011) Interferon lambda: a new sword in cancer immunotherapy. Clin Dev Immunol 2011:349575–349575. https://doi.org/10.1155/2011/349575
Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M Jr, Klein RS, Diamond MS (2015a) Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med 7(284):284ra259-284ra259
Lazear HM, Nice TJ, Diamond MS (2015b) Interferon-λ: immune functions at barrier surfaces and beyond. Immunity 43(1):15–28
Li M, He S (2006) Purification and characterization of recombinant human interleukin-29 expressed in Escherichia coli. J Biotechnol 122(3):334–340. https://doi.org/10.1016/j.jbiotec.2005.11.019
Li M, Huang D (2007) On-column refolding purification and characterization of recombinant human interferon-λ1 produced in Escherichia coli. Protein Expr Purif 53(1):119–123
Li Q, Kawamura K, Ma G, Iwata F, Numasaki M, Suzuki N, Shimada H, Tagawa M (2010) Interferon-λ induces G1 phase arrest or apoptosis in oesophageal carcinoma cells and produces anti-tumour effects in combination with anti-cancer agents. Eur J Cancer 46(1):180–190
Li JF, Cui XW, Ji HY, Qiu T, Ji XM, Du MX, Wu HT, Xu XZ, Zhang SQ (2011) High efficient expression of bioactive human BMP-14 in E. coli using SUMO fusion partner. Protein J 30(8):592–597
Mahlakoiv T, Ritz D, Mordstein M, DeDiego ML, Enjuanes L, Müller MA, Drosten C, Staeheli P (2012) Combined action of type I and type III interferon restricts initial replication of severe acute respiratory syndrome coronavirus in the lung but fails to inhibit systemic virus spread. J Gen Virol 93(12):2601–2605
Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR (2004) SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics 5(1):75–86
Marblestone JG, Edavettal SC, Lim Y, Lim P, Zuo X, Butt TR (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15(1):182–189. https://doi.org/10.1110/ps.051812706
Middelberg AP (2002) Preparative protein refolding. Trends Biotechnol 20(10):437–443
Muir AJ, Arora S, Everson G, Flisiak R, George J, Ghalib R, Gordon SC, Gray T, Greenbloom S, Hassanein T (2014) A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J Hepatol 61(6):1238–1246
Müller S, Hoege C, Pyrowolakis G, Jentsch S (2001) SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol 2(3):202–210
Nguyen T-Q, Duong T-H, Dang T-N-H, Le N-G, Le Q-G, Do T-H, Nguyen V-D, Le T-T-H, Truong N-H (2018) Enhanced soluble expression and effective purification of recombinant human interleukin-11 by SUMO fusion in Escherichia coli. Indian J Biotechnol 17:579–585
Palma-Ocampo HK, Flores-Alonso JC, Vallejo-Ruiz V, Reyes-Leyva J, Flores-Mendoza L, Herrera-Camacho I, Rosas-Murrieta NH, Santos-López G (2015) Interferon lambda inhibits dengue virus replication in epithelial cells. Virol J 12(1):1–14
Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I (2013) A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 45(2):164–171
Prokunina-Olsson L, Alphonse N, Dickenson RE, Durbin JE, Glenn JS, Hartmann R, Kotenko SV, Lazear HM, O’Brien TR, Odendall C (2020) COVID-19 and emerging viral infections: The case for interferon lambda. J Exp Med. https://doi.org/10.1084/jem.20200653
Rabhi-Essafi I, Sadok A, Khalaf N, Fathallah DM (2007) A strategy for high-level expression of soluble and functional human interferon α as a GST-fusion protein in E. coli. Protein Eng Des Sel 20(5):201–209
Rahman ZU, Ahmed N, Fazal N, Khan MI, Khan MA, Tahir S, Akram M, Ullah S, Zafar AU (2023) Enhancing the expression and purification of IL-29: a study of autoinduction and one-step purification methods. Advancements Life Sci 10(1):122–128
Robek MD, Boyd BS, Chisari FV (2005) Lambda interferon inhibits hepatitis B and C virus replication. J Virol 79(6):3851–3854
Shaldzhyan A, Zabrodskaya Y, Yolshin N, Kudling T, Lozhkov A, Plotnikova M, Ramsay E, Taraskin A, Nekrasov P, Grudinin M, Vasin A (2021) Clean and folded: production of active, high quality recombinant human interferon-λ1. Process Biochem 111:32–39. https://doi.org/10.1016/j.procbio.2021.08.029
Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J (2003) IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4(1):63–68
Sommereyns C, Paul S, Staeheli P, Michiels T (2008) IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 4(3):e1000017
Stewart EJ, Åslund F, Beckwith J (1998) Disulfide bond formation in the Escherichia coli cytoplasm: an in vivo role reversal for the thioredoxins. EMBO J 17(19):5543–5550
Tezuka Y, Endo S, Matsui A, Sato A, Saito K, Semba K, Takahashi M, Murakami T (2012) Potential anti-tumor effect of IFN-λ2 (IL-28A) against human lung cancer cells. Lung Cancer 78(3):185–192
Wang H, Xiao Y, Fu L, Zhao H, Zhang Y, Wan X, Qin Y, Huang Y, Gao H, Li X (2010) High-level expression and purification of soluble recombinant FGF21 protein by SUMO fusion in Escherichia coli. BMC Biotechnol 10(1):1–9
Wang D, Fang L, Zhao F, Luo R, Chen H, Xiao S (2011) Molecular cloning, expression and antiviral activity of porcine interleukin-29 (poIL-29). Dev Comp Immunol 35(3):378–384
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22(11):1393–1398
Xie YF, Chen H, Huang BR (2007) Expression, purification and characterization of human IFN-λ1 in Pichia pastoris. J Biotechnol 129(3):472–480. https://doi.org/10.1016/j.jbiotec.2007.01.018
Zitzmann K, Brand S, Baehs S, Göke B, Meinecke J, Spöttl G, Meyer H, Auernhammer CJ (2006) Novel interferon-λs induce antiproliferative effects in neuroendocrine tumor cells. Biochem Biophys Res Commun 344(4):1334–1341
Funding
This research received no specific funds, grants, or other support from any funding agency in the public, commercial, or not-for-profit sectors during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AM: Research Design, Manuscript Writing. NA: Idea Conception, Research Supervision. MA: Data Analysis, Manuscript Reviewer. NAF: Data Collection and Analysis. ST: Data Collection, Manuscript Review. KM: Critical Review.
Corresponding author
Ethics declarations
Conflict of interest
The authors (s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
This article does not contain any studies involving human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Munir, A., Ahmed, N., Akram, M. et al. Enhanced soluble expression of active recombinant human interleukin-29 using champion pET SUMO system. Biotechnol Lett 45, 1001–1011 (2023). https://doi.org/10.1007/s10529-023-03402-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-023-03402-x