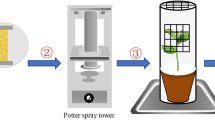
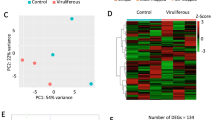
Abstract
One of the successful strategies developed for studying the gene function of aphids is to silence aphid gene expression by plant-mediated RNA interference (RNAi). In this study, we analyzed the expression patterns and the biological functions of genes related to chitin metabolism ApCht7 and ApCht10 by using gene-specific plant-mediated RNAi in the green pea aphid, Acyrthosiphon pisum (Ap). The RT-qPCR results demonstrated that the RNAi-mediated silencing of these ApChts suppressed the expression of most genes involved in the chitin degradation pathway, but enhanced the expressions of ApHK, ApGFAT, ApPGM and ApCHS, indicating that the RNAi of ApChts could affect the expression of genes related to chitin metabolism and regulate the chitin metabolism. Furthermore, it resulted in a decrease in aphid body weight of aphids, with mortality rates ranging from 3.3 to 26.1%. Abnormal phenotypes (e.g., molting defect and wrinkled integument) were observed in the treated nymphs, leading to early lethality. The aphid reproduction rate reduced by 27.9–40.5% at 120 h after RNAi of ApChts. The target genes ApCht7 and ApCht10 expression were also downregulated in the progeny nymphs born from the mothers exposed to dsRNA-plants with significantly lower body weights. The previously mentioned results suggest that the effects of RNAi ApCht7 and ApCht10 are heritable and passed down to offspring. Our study provides a foundation for the practical application of the plant-mediated RNAi of ApCht7 and ApCht10 as a powerful tools and methods for controlling field aphid outbreaks.






Similar content being viewed by others
Data availability
The materials and data in the current study are available from the corresponding author on reasonable request.
References
Arakane Y, Muthukrishnan S (2010) Insect chitinase and chitinase-like proteins. Cell Mol Life Sci 67:201–216. https://doi.org/10.1007/s00018-009-0161-9
Abdellatef E, Will T, Koch A, Imani J, Vilcinskas A, Kogel KH (2015) Silencing the expression of the salivary sheath protein causes transgenerational feeding suppression in the aphid Sitobion avenae. Plant Biotechnol J 13:849–857. https://doi.org/10.1111/pbi.12322
Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M (2007) Control of coleopteran insect pests through RNA interference. Nat Biotechnol 25:1322–1326. https://doi.org/10.1038/nbt1359
Blattner R, Gerard PJ, Spindler-Barth M (1997) Synthesis and biological activity of allosamidin and allosamidin analogues. Pestic Sci 50:312–318. https://doi.org/10.1002/(SICI)1096-9063(199708)50:4%3c312::AID-PS594%3e3.0.CO;2-O
Chen C, Yang H, Tang B, Yang WJ, Jin DC (2017) Identification and functional analysis of chitinase 7 gene in white-backed planthopper, Sogatella furcifera. Comp Biochem Physiol b: Biochem Mol Biol 208–209:19–28. https://doi.org/10.1016/j.cbpb.2017.03.002
Chen J, Li T, Pang R (2020) miR-2703 regulates the chitin biosynthesis pathway by targeting chitin synthase 1a in Nilaparvata lugens. Insect Mol Biol 29:38–47. https://doi.org/10.1111/imb.12606
Coleman AD, Wouters RHM, Mugford ST, Hogenhout SA (2015) Persistence and transgenerational effect of plant-mediated RNAi in aphids. J Exp Bot 66:541–548. https://doi.org/10.1093/jxb/eru450
Ding BY, Niu J, Shang F, Yang L, Wang JJ (2020) Parental silencing of a horizontally transferred carotenoid desaturase gene causes a reduction of red pigment and fitness in the pea aphid. Pest Manag Sci 76:2423–2433. https://doi.org/10.1002/ps.5783
Dong W, Gao YH, Zhang XB, Moussian B, Zhang JZ (2020) Chitinase10 controls chitin amounts and organization in the wing cuticle of Drosophila. Insect Science 27:1198–1207. https://doi.org/10.1111/1744-7917.12774
Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10:94–108. https://doi.org/10.1038/nrg2504
Grimson A, Srivastava M, Fahey B, Woodcroft BJ, Chiang HR, King N, Degnan BM, Rokhsar DS, Bartel DP (2008) Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 455:1193–1197. https://doi.org/10.1038/nature07415
Harrington CD (1945) Biological races of the pea aphid. J Econ Entomol 38:12–22. https://doi.org/10.1093/jee/38.1.12
Hannon GJ (2002) RNA interference. Nature 418:244–251. https://doi.org/10.1038/418244a
Jiang Z, Khan SA, Heckel DG, Bock R (2017) Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol 35:871–882. https://doi.org/10.1016/j.tibtech.2017.04.009
Kramer KJ, Corpuz L, Choi HK, Muthukrishnan S (1993) Sequence of a cDNA and expression of the gene encoding epidermal and gut chitinases of Manduca sexta. Insect Biochem Mol Biol 23:691–701. https://doi.org/10.1016/0965-1748(93)90043-R
Koch A, Kogel KH (2014) New wind in the Sails: improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol J 12:821–831. https://doi.org/10.1111/pbi.12226
Kola VSR, Renuka P, Madhav MS, Mangrauthia SK (2015) Key enzymes and proteins of crop insects as candidate for RNAi based gene silencing. Front Physiol 6:119. https://doi.org/10.3389/fphys.2015.00119
Li CC, Ul Haq I, Khurshid A, Tao Y, Quandahor P, Zhou JJ, Liu CZ (2022) Effects of abiotic stresses on the expression of chitinase-like genes in Acyrthosiphon pisum. Front Physiol 13:1024136. https://doi.org/10.3389/fphys.2022.1024136
Li DQ, Zhang JQ, Wang Y, Liu XJ, Ma E, Sun Y, Li S, Zhu KY, Zhang JZ (2015) Two chitinase 5 genes from Locusta migratoria: molecular characteristics and functional differentiation. Insect Biochem Mol Biol 58:46–54. https://doi.org/10.1016/j.ibmb.2015.01.004
Li T, Chen J, Fan X, Chen W, Zhang W (2017) MicroRNA and dsRNA targeting chitin synthase a reveal a great potential for pest management of the hemipteran insect Nilaparvata lugens. Pest Manag Sci 73:1529–1537. https://doi.org/10.1002/ps.4492
Liu SH, Li HF, Yang Y, Yang RL, Yang WJ, Jiang HB, Dou W, Smagghe G, Wang JJ (2018) Genome-wide identification of chitinase and chitin deacetylase gene families in the oriental fruit fly, Bactrocera dorsalis (Hendel). Comparative biochemistry and physiology. Part D Genom Proteom 27D:13–22. https://doi.org/10.1016/j.cbd.2018.04.005
Liu SH, Xia YD, Zhang Q, Li W, Li RY, Liu Y, Chen EH, Dou W, Stelinski LL, Wang JJ (2020) Potential targets for controlling Bactrocera dorsalis using cuticle- and hormone-related genes revealed by a developmental transcriptome analysis. Pest Manag Sci 76:2127–2143. https://doi.org/10.1002/ps.5751
Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 25:1307–1313. https://doi.org/10.1038/nbt1352
Mbiza NIT, Feng WZ, Xia PL, Zhang HR, Hu ZW, Chen Q, Zeng M, Zhang Y, Yang YZ, Li CR, Zhang JM (2021) Insect chitinase: molecular biology and the potential roles in insect pest management. Afr J Agric Food Sci 4:1–11
Mello CC, Conte D (2004) Revealing the world of RNA interference. Nature 431:338–342. https://doi.org/10.1038/nature02872
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412. https://doi.org/10.1242/jeb.00709
Morishita M, Azuma K (1990) Decreased susceptibilities of the green peach aphid (Myzus persicae Sulzer) to some synthetic pyrethroids. Jpn J Appl Entomol Zool 34:163–165. https://doi.org/10.1303/jjaez.34.163
Muthukrishnan S, Merzendorfer H, Arakane Y, Yang Q (2019) Chitin organizing and modifying enzymes and proteins involved in remodeling of the insect cuticle. Adv Exp Med Biol 1142:83–114. https://doi.org/10.1007/978-981-13-7318-3_5
Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y (2018) A chitinase with two catalytic domains is required for organization of the cuticular extracellular matrix of a beetle. PLoS Genet 14:e1007307. https://doi.org/10.1371/journal.pgen.1007307
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811. https://doi.org/10.1038/35888
Omar MAA, Ao Y, Li M, He K, Xu L, Tong HJ (2019) The functional difference of eight chitinase genes between male and female of the cotton mealybug, Phenacoccus solenopsis. Insect Mol Biol 28:550–567. https://doi.org/10.1111/imb.12572
Pesch YY, Riedel D, Patil KR, Loch G, Behr M (2015) Chitinases and Imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects. Sci Rep 6:18340. https://doi.org/10.1038/srep18340
Pitino M, Hogenhout SA (2013) Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol Plant Microbe Interact 26:130–139. https://doi.org/10.1094/MPMI-07-12-0172-FI
Pitino M, Coleman AD, Maffei ME, Ridout CJ, Hogenhout SA (2011) Silencing of aphid genes by dsRNA feeding from plants. PLoS ONE 6:e25709. https://doi.org/10.1371/journal.pone.0025709
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007. https://doi.org/10.1093/nar/29.9.e45
Ryalls JMW, Riegler M, Moore BD, Johnson SN (2013) Biology and trophic interactions of lucerne aphids. Agric for Entomol 15:335–350. https://doi.org/10.1111/afe.12024
Quan GX, Ladd T, Duan J, Wen FY, Doucet D, Cusson M, Krell PJ (2013) Characterization of a spruce budworm chitin deacetylase gene: stage-and tissue-specific expression, and inhibition using RNA interference. Insect Biochem Mol Biol 43:683–691. https://doi.org/10.1016/j.ibmb.2013.04.005
Sakuda S, Isogai A, Matsumoto S, Suzuki A (1987) Search for microbial insect growth regulators II allosamidin, a novel insect chitinase inhibitor. J Antibiot 40:296–300. https://doi.org/10.7164/antibiotics.40.296
Shang F, Ding BY, Ye C, Yang L, Chang TY, Xie JQ, Tang LD, Niu JZ, Wang JJ (2019) Evaluation of a cuticle protein gene as a potential RNAi target in aphids. Pest Manag Sci 76:134–140. https://doi.org/10.1002/ps.5599
Shang F, Niu J, Ding BY, Zhang W, Wang JJ (2020) The mir-9b microRNA mediates dimorphism and development of wing in aphids. Proc Natl Acad Sci USA 117:8404–8409. https://doi.org/10.1073/pnas.1919204117
Shao ZM, Li YJ, Zhang XR, Chu J, Ma JH, Liu ZX, Wang J, Sheng S, Wu FA (2020) Identification and functional study of chitin metabolism and detoxification-related genes in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) based on transcriptome analysis. Int J Mol Sci 21:1904. https://doi.org/10.3390/ijms21051904
Tang B, Qin Z, Shi ZK, Wang S, Guo XJ, Wang SG, Zhang F (2014) Trehalase in Harmonia axyridis (Coleoptera: Coccinellidae): effects on beetle locomotory activity and the correlation with trehalose metabolism under starvation conditions. Appl Entomol Zool 49:255–264. https://doi.org/10.1007/s13355-014-0244-4
Tang B, Zhang L, Xiong XP, Wang HJ, Wang SG (2018) Advances in trehalose metabolism and its regulation of insect chitin synthesis. Scient Agric Sin 51:697–707
Wang G, Gou YP, Guo SF, Zhou JJ, Liu CZ (2021) RNA interference of trehalose-6-phosphate synthase and trehalase genes regulates chitin metabolism in two color morphs of Acyrthosiphon pisum Harris. Sci Rep 11:948. https://doi.org/10.1038/s41598-020-80277-2
Wang M, Thomas N, Jin H (2017) Cross-kingdom RNA trafficking and environmental RNAi for powerful innovative pre-and post-harvest plant protection. Curr Opin Plant Biol 38:133–141. https://doi.org/10.1016/j.pbi.2017.05.003
Yan XP, Zhao D, Zhang YK, Guo W, Wang W, Zhao KL, Gao YJ, Wang XY (2018) Identification and characterization of chitin deacetylase2 from the American white moth, Hyphantria cunea (Drury). Gene 670:98–105. https://doi.org/10.1016/j.gene.2018.05.069
Yang MM, Zhao LN, Shen QD, Xie GQ, Wang SG, Tang B (2016) Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest Manag Sci 73:206–216. https://doi.org/10.1002/ps.4287
Yang F, Chen S, Dai ZM, Chen DF, Duan RB, Wang HL, Jia SN, Yang WJ (2013) Regulation of trehalase expression inhibits apoptosis in diapause cysts of Artemia. Biochem J 456:185–194. https://doi.org/10.1042/BJ20131020
Yang WQ, Fan D (2018) Cloning, expression and ecdysone regulation of chitinase MsCHT7 from the oriental armyworm, Mythimna separata walker. Chin J BiolControl 34:354–363. https://doi.org/10.16409/j.cnki.2095-039x.2018.03.004
Ye C, Wang ZW, Sheng YL, Wang ZG, Smagghe G, Christiaens O, Niu JZ, Wang JJ (2021) GNBP1 as a potential RNAi target to enhance the virulence of Beauveria bassiana for aphid control. J Pest Sci 95:539–550. https://doi.org/10.1007/s10340-021-01388-x
Ye C, Jiang YD, An X, Yang L, Shang F, Niu JZ, Wang JJ (2019) Effects of RNAi-based silencing of chitin synthase gene on moulting and fecundity in pea aphids (Acyrthosiphon pisum). Sci Rep 9:3694. https://doi.org/10.1038/s41598-019-39837-4
Yu XD, Liu ZC, Huang SL, Chen ZQ, Sun YW, Duan PF, Ma YZ, Xia LQ (2016) RNAi-mediated plant protection against aphids. Pest Manag Sci 72:1090–1098. https://doi.org/10.1002/ps.4258
Zhang J, Khan SA, Heckel DG, Bock R (2017) Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol 35:871–882. https://doi.org/10.1016/j.tibtech.2017.04.009
Zhang JH, Li HY, Zhong X, Tian JF, Segers A, Xia LQ, Francis F (2022) RNA-interference-mediated aphid control in crop plants: a review. Agriculture 12:2108
Zhao Y, Park RD, Muzzarelli RA (2010) Chitin deacetylases: properties and applications. Mar Drugs 14:24–46. https://doi.org/10.3390/md8010024
Zhu B, Shan J, Li R, Liang P, Gao XW (2019) Identification and RNAi-based function analysis of chitinase family genes in diamondback moth, Plutella xylostella. Pest Manag Sci 75:1951–1961. https://doi.org/10.1002/ps.5308
Zhu KY, Merzendorfer H, Zhang WQ, Zhang JZ, Muthukrishnan S (2016) Biosynthesis, turnover, and functions of chitin in insects. Annu Rev Entomol 61:177–196. https://doi.org/10.1146/annurev-ento-010715-023933
Zhu QS, Arakane Y, Beeman RW, Beeman RW, Kramer KJ, Muthukrishnan S (2008) Functional specialization among insect chitinase family genes revealed by RNA interference. Proc Natl Acad Sci USA 105:6650–6655. https://doi.org/10.1073/pnas.0800739105
Acknowledgements
We would like to thank all of the participants who volunteered their time in the study. We appreciate very much the valuable comments and suggestion by the Reviewers and Editors.
Funding
This research was funded by the University Innovation Fund Project (No. 2023A-057), Scientific Research Start-up Funds for Openly-recruited Doctors of Gansu Agricultural University (GAU-KYQD-2022-26), and National Science Foundation of China (No. 31960351, 31960227).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
Not applicable.
Consent for publication
All authors consent to the publication of this manuscript in the Journal of Pest Science.
Additional information
Communicated by Swevers Luc.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Wang, L., Liu, L. et al. Silencing of ApCht7 and ApCht10 revealed their function and evaluation of their potential as RNAi targets in Acyrthosiphon pisum. J Pest Sci 97, 1123–1134 (2024). https://doi.org/10.1007/s10340-023-01722-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01722-5