Abstract
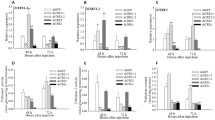
RNA interference (RNAi) is an efficient, species-specific technology to control invasive pests. However, its efficacy varies among insect species, notably due to the presence of double-stranded RNA degrading enzymes (dsRNases) in the midgut and hemolymph. These dsRNases degrade the gene-specific exogenous double-stranded ribonucleic acid (dsRNA) before it can bind to its target transcript. Here, we explored the role of dsRNases in RNAi efficacy in Zeugodacus cucurbitae, a major insect pest of cucurbit plants. Our RNAi gene target was the Coatomer subunit alpha (ZcCOPI-alpha), which plays a major role in insect development and survival. We identified two dsRNases (ZcdsRNase1, ZcdsRNase2) using a bioinformatics analysis. Using qRT-PCR, we showed that their expression level was highest in Z. cucurbitae’s midgut and during the larval stage, compared to the lower levels measured in other tissue and other developmental stages. We therefore co-silenced the gene of each dsRNase together with ZcCOPI-alpha in third instar larvae. Co-silencing ZcdsRNase1 along with ZcCOPI-alpha led to 84% larval mortality, while co-silencing ZcdsRNase2 along with ZcCOPI-alpha caused 44% of larval mortality only, and silencing ZcCOPI-alpha alone caused 59% of larval mortality. Co-silencing ZcdsRNase1 reduced dsZcCOPI-alpha degradation and improved RNAi efficacy in Z. cucurbitae. We also showed that ZcCOPI-alpha knockdown severely affected the survival and development of Z. cucurbitae and caused ovarian abnormalities. The demonstrated co-silencing approach offers interesting avenues for the industrial development of RNAi-based pest control strategies targeting Z. cucurbitae.





Similar content being viewed by others
Data availability
Data are made available upon request to authors.
References
Agrawal N, Dasaradhi PVN, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK (2003) RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev 67:657–685. https://doi.org/10.1128/mmbr.67.4.657-685.2003
Ahmad S, Jamil M, Fahim M, Zhang S, Ullah F, Lyu B, Luo Y (2021) RNAi-mediated knockdown of imaginal disc growth factors (IDGFs) genes causes developmental malformation and mortality in melon fly, Zeugodacus cucurbitae. Front Genet. https://doi.org/10.3389/fgene.2021.691382
Alamalakala L, Parimi S, Patel N, Char B (2018) Insect RNAi: integrating a new tool in the crop protection toolkit. In: Kumar D, Gong C (eds) Trends in insect molecular biology and biotechnology. Springer International Publishing, Cham, pp 193–232. https://doi.org/10.1007/978-3-319-61343-7_10
Allen ML, Walker WB (2012) Saliva of Lygus lineolaris digests double stranded ribonucleic acids. J Insect Physiol 58:391–396. https://doi.org/10.1016/j.jinsphys.2011.12.014
Baum JA, Roberts JK (2014) Progress towards RNAi-mediated insect pest management. In: Dhadialla TS, Gill P (eds) Advances in insect physiology. Academic Press, pp 249–295. https://doi.org/10.1016/B978-0-12-800197-4.00005-1
Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, Johnson S, Plaetinck G, Munyikwa T, Pleau M, Vaughn T, Roberts J (2007) Control of coleopteran insect pests through RNA interference. Nat Biotech 25:1322–1326. https://doi.org/10.1038/nbt1359
Bellés X (2010) Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu Rev Entomol 55:111–128. https://doi.org/10.1146/annurev-ento-112408-085301
Bramlett M, Plaetinck G, Maienfisch P (2020) RNA-based biocontrols—a new paradigm in crop protection. Engineering 6:522–527. https://doi.org/10.1016/j.eng.2019.09.008
Cappelle K, De Oliveira CFR, Van Eynde B, Christiaens O, Smagghe G (2016) The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado potato beetle midgut. Insect Mol Biol 25:315–323. https://doi.org/10.1111/imb.12222
Christiaens O, Swevers L, Smagghe G (2014) DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 53:307–314. https://doi.org/10.1016/j.peptides.2013.12.014
Cooper AMW, Silver K, Zhang J, Park Y, Zhu KY (2019) Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag Sci 75:18–28. https://doi.org/10.1002/ps.5126
Damalas CA, Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health 8:1402–1419. https://doi.org/10.3390/ijerph8051402
De Meyer M, Delatte H, Mwatawala M, Quilici S, Vayssières JF, Virgilio M (2015) A review of the current knowledge on Zeugodacus cucurbitae (Coquillett) (Diptera, tephritidae) in Africa, with a list of species included in Zeugodacus. ZooKeys 540:539–557. https://doi.org/10.3897/zookeys.540.9672
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Fan YH, Song HF, Abbas M, Wang YL, Li T, Ma EB, Cooper AMW, Silver K, Zhu KY, Zhang JZ (2021) A dsRNA-degrading nuclease (dsRNase2) limits RNAi efficiency in the Asian corn borer (Ostrinia furnacalis). Insect Sci 28:1677–1689. https://doi.org/10.1111/1744-7917.12882
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811. https://doi.org/10.1038/35888
Ge T, Huang X, Pan X, Zhang J, Xie R (2019) Genome-wide identification and expression analysis of citrus fruitlet abscission-related polygalacturonase genes. Biotech 9:250. https://doi.org/10.1007/s13205-019-1782-9
Huvenne H, Smagghe G (2010) Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56:227–235. https://doi.org/10.1016/j.jinsphys.2009.10.004
Isoe J, Collins J, Badgandi H, Day WA, Miesfeld RL (2011) Defects in coatomer protein I (COPI) transport cause blood feeding-induced mortality in Yellow Fever mosquitoes. Proc Natl Acad Sci 108:E211–E217. https://doi.org/10.1073/pnas.1102637108
Jamil M, Ahmad S, Ran Y, Ma S, Cao F, Lin X, Yan R (2022) Argonaute1 and gawky are required for the development and reproduction of melon fly, Zeugodacus cucurbitae. Front Genet. https://doi.org/10.3389/fgene.2022.880000
Jin S, Singh ND, Li L, Zhang X, Daniell H (2015) Engineered chloroplast dsRNA silences cytochrome p450 monooxygenase, V-ATPase and chitin synthase genes in the insect gut and disrupts Helicoverpa armigera larval development and pupation. Plant Biotech J 13:435–446. https://doi.org/10.1111/pbi.12355
Kumar A, Sahoo SK (2021) Biointensive integrated pest management. CRC Press. https://doi.org/10.1201/9781003256922-3
Letunic I, Bork P (2011) Interactive tree of life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acid Res 39:W475–W478. https://doi.org/10.1093/nar/gkr201
Li WJ, Song YJ, Xu HQ, Wei D, Wang JJ (2021) Vitelline membrane protein gene ZcVMP26Ab and its role in preventing water loss in Zeugodacus cucurbitae (Coquillett) embryos. Entomol Gen 41:279–288. https://doi.org/10.1127/entomologia/2021/1037
Li R, Zhu B, Liang P, Gao XW (2022) Identification of carboxylesterase genes contributing to multi-insecticide resistance in Plutella xylostella (L.). Entomol Gen 42:967–976. https://doi.org/10.1127/entomologia/2022/1572
Liu J, Swevers L, Iatrou K, Huvenne H, Smagghe G (2012) Bombyx mori DNA/RNA non-specific nuclease: expression of isoforms in insect culture cells, subcellular localization and functional assays. J Insect Physiol 58:1166–1176. https://doi.org/10.1016/j.jinsphys.2012.05.016
Liu X, Lin X, Li J, Li F, Cao F, Yan R (2020) A novel solid artificial diet for Zeugodacus cucurbitae (Diptera: Tephritidae) larvae with fitness parameters assessed by two-sex life table. J Insect Sci 20:21. https://doi.org/10.1093/JISESA/IEAA058
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Luo Y, Wang X, Wang X, Yu D, Chen B, Kang L (2013) Differential responses of migratory locusts to systemic RNA interference via double-stranded RNA injection and feeding. Insect Mol Biol 22:574–583. https://doi.org/10.1111/imb.12046
Luo Y, Chen Q, Luan J, Chung SH, Van Eck J, Turgeon R, Souglas AE (2017) Towards an understanding of the molecular basis of effective RNAi against a global insect pest, the whitefly Bemisia tabaci. Insect Biochem Mol Biol 88:21–29. https://doi.org/10.1016/j.ibmb.2017.07.005
Ma M, Zhang YX, Chen D, Smagghe G, Wang JJ, Wei D (2022) Functional characterization of a glutathione S-transferase gene GSTe10 that contributes to ovarian development in Bactrocera dorsalis (Hendel). Entomol Gen 42:539–547. https://doi.org/10.1127/entomologia/2022/1363
Machado V, Rodríguez-García MJ, Sánchez-García FJ, Galián J (2014) RNA interference: a new strategy in the evolutionary arms race between human control strategies and insect pests. Folia Biol 62:335–343. https://doi.org/10.3409/fb62_4.335
Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nature Biotech 25:1307–1313. https://doi.org/10.1038/nbt1352
Mcquate GT, Teruya T (2015) Melon fly, Bactrocera Cucurbitae (Diptera: Tephritidae), infestation in host fruits in the southwestern Islands of Japan before the initiation of island-wide population suppression, as recorded in publications of Japanese public institutions. Int J Insect Sci 7:IJIS.S24582. https://doi.org/10.4137/ijis.s24582
Mkiga AM, Mwatawala MW, Ewer J (2015) Developmental biology of Zeugodacus cucurbitae (Diptera: Tephritidae) in three cucurbitaceous hosts at different temperature regimes. J Insect Sci 15:160. https://doi.org/10.1093/jisesa/iev141
Mwatawala MW, De Meyer M, Makundi RH, Maerere AP (2009) Host range and distribution of fruit-infesting pestiferous fruit flies (Diptera, Tephritidae) in selected areas of Central Tanzania. Bull Entomol Res 99:629–641. https://doi.org/10.1017/S0007485309006695
Palma-Onetto V, Oliva D, González-Teuber M (2021) Lethal and oxidative stress side effects of organic and synthetic pesticides on the insect scale predator Rhyzobius lophanthae. Entomol Gen 41:345–355. https://doi.org/10.1127/entomologia/2021/1045
Peng Y, Wang K, Zhu G, Han Q, Chen J, Elzaki MEA, Sheng C, Zhao C, Palli SR, Han Z (2020a) Identification and characterization of multiple dsRNases from a lepidopteran insect, the tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Pestic Biochem Physiol 162:86–95. https://doi.org/10.1016/j.pestbp.2019.09.011
Peng Y, Wang K, Chen J, Wang J, Zhang H, Ze L, Zhu G, Zhao C, Xiao H, Han Z (2020b) Identification of a double-stranded RNA-degrading nuclease influencing both ingestion and injection RNA interference efficiency in the red flour beetle Tribolium castaneum. Insect Biochem Mol Biol 125:10344. https://doi.org/10.1016/j.ibmb.2020.103440
Pires Paula DP, Lozano RE, Menger JP, Andow DA, Koch RL (2021) Identification of point mutations related to pyrethroid resistance in voltage-gated sodium channel genes in Aphis glycines. Entomol Gen 41:243–255. https://doi.org/10.1127/entomologia/2021/1226
Prentice K, Christiaens O, Pertry I, Bailey A, Niblett C, Ghislain M, Gheysen G, Smagghe G (2017) RNAi-based gene silencing through dsRNA injection or ingestion against the African sweet potato weevil Cylas puncticollis (Coleoptera: Brentidae). Pest Manag Sci 73:44–52. https://doi.org/10.1002/ps.4337
Price DRG, Gatehouse JA (2008) RNAi-mediated crop protection against insects. Trends Biotech 26:393–400. https://doi.org/10.1016/j.tibtech.2008.04.004
Rank AP, Koch A (2021) Lab-to-field transition of RNA Spray applications – How far are we? Front Plant Sci. https://doi.org/10.3389/fpls.2021.755203
Ren D, Cai Z, Song J, Wu Z, Zhou S (2014) DsRNA uptake and persistence account for tissue-dependent susceptibility to RNA interference in the migratory locust, Locusta migratoria. Insect Mol Biol 23:175–184. https://doi.org/10.1111/imb.12074
Rodrigues TB, Figueira A (2016) Management of insect pest by RNAi — a new tool for crop protection. RNA Interf. https://doi.org/10.5772/61807
Shafiq Ansari M, Hasan F, Ahmad N (2012) Threats to fruit and vegetable crops: fruit flies (Tephritidae) – ecology, behaviour, and management. J Crop Sci Biotech 15:169–188. https://doi.org/10.1007/s12892-011-0091-6
Sharma R, Christiaens O, Taning CNT, Smagghe G (2021a) RNAi-mediated mortality in southern green stinkbug Nezara viridula by oral delivery of dsRNA. Pest Manag Sci 77:77–84. https://doi.org/10.1002/ps.6017
Sharma R, Taning CN, Smagghe G, Christiaens O (2021b) Silencing of double-stranded ribonuclease improves oral RNAi Efficacy in southern green stinkbug Nezara viridula. InSects. https://doi.org/10.3390/insects12020115
Shukla JN, Kalsi M, Sethi A, Narva KE, Fishilevich E, Singh S, Mogilicherla K, Palli SR (2016) Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol 13:656–669. https://doi.org/10.1080/15476286.2016.1191728
Singh IK, Singh S, Mogilicherla K, Shukla JN, Palli SR (2017) Comparative analysis of double-stranded RNA degradation and processing in insects. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-17134-2
Sohail S, Tariq K, Zheng W, Ali MW, Peng W, Raza MF, Zhang H (2019) RNAi-mediated knockdown of tssk1 and tektin1 genes impair male fertility in Bactrocera dorsalis. InSects. https://doi.org/10.3390/insects10060164
Song H, Fan Y, Zhang J, Cooper AMW, Silver K, Li D, Li T, Ma E, Zhu KY, Zhang J (2019) Contributions of dsRNases to differential RNAi efficiencies between the injection and oral delivery of dsRNA in Locusta migratoria. Pest Manag Sci 75:1707–1717. https://doi.org/10.1002/ps.5291
Song H, Zhang J, Li D, Cooper AMW, Silver K, Li T, Liu X, Ma E, Zhu KY, Zhang J (2017) A double-stranded RNA degrading enzyme reduces the efficiency of oral RNA interference in migratory locust. Insect Biochem Mol Biol 86:68–80. https://doi.org/10.1016/j.ibmb.2017.05.008
Spit J, Philips A, Wynant N, Santos D, Plaetinck G, Vanden Broeck J (2017) Knockdown of nuclease activity in the gut enhances RNAi efficiency in the Colorado potato beetle, Leptinotarsa decemlineata, but not in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 81:103–116. https://doi.org/10.1016/j.ibmb.2017.01.004
Stonehouse JM, Singh HS, Patel RK, Satpathy S, Shivalingaswamy TM, Rai S, Verghese A, Mumford JD (2007) The measurement and modelling of losses of cucurbits to tephritid fruit flies. Commun Biometry Crop Sci 2:17–25
Taning CNT, Christiaens O, Berkvens N, Casteels H, Maes M, Smagghe G (2016) Oral RNAi to control Drosophila suzukii: laboratory testing against larval and adult stages. J Pest Sci 89:803–814. https://doi.org/10.1007/s10340-016-0736-9
Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, Smagghe G (2011) RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol 57:231–245. https://doi.org/10.1016/j.jinsphys.2010.11.006
Tian Z, Guo S, Zhu F, Liu W, Wang XP (2022) Targeting coat protein II complex genes via RNA interference inhibits female adult feeding and reproductive development in the cabbage beetle Colaphellus bowringi. Pest Manag Sci 78:2141–2150. https://doi.org/10.1002/ps.6836
Tudi M, Ruan HD, Wang L, Lyu J, Sadler R, Connell D, Chu C, Phung DT (2021) Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health 18:1–24. https://doi.org/10.3390/ijerph18031112
Ullah M, Das G, Islam K (2008) Biology and host suitability of cucurbit fruit fly, Bactrocera cucurbitae (coq.): a comparative study on five different cucurbit species. J Agrofor Environ 2:1–8
Ullah F, Gul H, Tariq K, Hafeez M, Desneux N, Gao X, Song D (2022) RNA interference-mediated silencing of ecdysone receptor (EcR) gene causes lethal and sublethal effects on melon aphid, Aphis gossypii. Entomol Gen 42:791–797. https://doi.org/10.1127/entomologia/2022/1434
Wang K, Peng Y, Pu J, Fu W, Wang J, Han Z (2016) Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem Mol Biol 77:1–9. https://doi.org/10.1016/j.ibmb.2016.07.007
Wang K, Peng Y, Fu W, Shen Z, Han Z (2019) Key factors determining variations in RNA interference efficacy mediated by different double-stranded RNA lengths in Tribolium castaneum. Insect Mol Biol 28:235–245. https://doi.org/10.1111/imb.12546
Willow J, Veromann E (2021) Highly variable dietary RNAi sensitivity among Coleoptera. Front Plant Sci. https://doi.org/10.3389/fpls.2021.790816
Willow J, Taning CNT, Cook SM, Sulg S, Silva AI, Smagghe G, Veromann E (2021) RNAi targets in agricultural pest insects: advancements, knowledge gaps, and IPM. Front Agron. https://doi.org/10.3389/fagro.2021.794312
Wynant N, Santos D, Verdonck R, Spit J, Van Wielendaele P, Vanden BJ (2014a) Identification, functional characterization and phylogenetic analysis of double stranded RNA degrading enzymes present in the gut of the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 46:1–8. https://doi.org/10.1016/j.ibmb.2013.12.008
Wynant N, Duressa TF, Santos D, Van Duppen J, Proost P, Huybrechts R, Vanden Broeck J (2014b) Lipophorins can adhere to dsRNA, bacteria and fungi present in the hemolymph of the desert locust: a role as general scavenger for pathogens in the open body cavity. J Insect Physiol 64:7–13. https://doi.org/10.1016/j.jinsphys.2014.02.010
Yang J, Han ZJ (2014) Efficiency of different methods for dsRNA delivery in cotton bollworm (Helicoverpa armigera). J Integr Agric 13:115–123. https://doi.org/10.1016/S2095-3119(13)60511-0
Yoon JS, Ahn SJ, Flinn CM, Choi MY (2021) Identification and functional analysis of dsRNases in spotted-wing drosophila, Drosophila suzukii. Arch Insect Biochem Physiol 107:e21822. https://doi.org/10.1002/arch.21822
Yu N, Christiaens O, Liu J, Niu J, Cappelle K, Caccia S, Huvenne H, Smagghe G (2013) Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect Sci 20:4–14. https://doi.org/10.1111/j.1744-7917.2012.01534.x
Zhang J, Khan SA, Hasse C, Ruf S, Heckel DG, Bock R (2015) Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 347:991–994. https://doi.org/10.1126/science.1261680
Zhang X, Fan Z, Wang Q, Kong X, Liu F, Fang J, Zhang S, Zhang Z (2022) RNAi efficiency through dsRNA Injection is enhanced by knockdown of dsRNA nucleases in the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int J Mol Sci. https://doi.org/10.3390/ijms23116182
Zhu KY, Palli SR (2020) Mechanisms, applications, and challenges of insect RNA interference. Annu Rev Entomol 65:293–311. https://doi.org/10.1146/annurev-ento-011019-025224
Funding
This research was funded by Hainan Provincial Natural Science Foundation Youth Fund Project (322QN254), Hainan Province Science and Technology Special Fund (ZDYF2022XDNY138, ZDKJ2021007), NSFC (32260685).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This article does not contain any experiment with human participants or animals performed by any of the authors.
Additional information
Communicated by Subba Palli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, S., Jamil, M., Jaworski, C.C. et al. Double-stranded RNA degrading nuclease affects RNAi efficiency in the melon fly, Zeugodacus cucurbitae. J Pest Sci 97, 397–409 (2024). https://doi.org/10.1007/s10340-023-01637-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01637-1