Abstract
Object
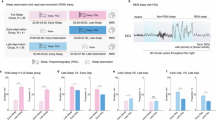
In humans, even a single night of partial sleep deprivation (PSD) can have a negative impact on cognition and affective processing, suggesting that sleep pressure represents a basic physiological constraint of brain function. Among the spontaneously fluctuating resting state networks, the default mode network (DMN) and its anticorrelated network (ACN) hold key functions in segregating internally and externally directed awareness. Task fMRI after sleep deprivation has revealed altered activation patterns in both networks. We hypothesized that effects of PSD in these intrinsically coupled networks can be detected by resting state fMRI.
Methods
We obtained 6-minute echoplanar imaging time series (1.5 Tesla) during eyes-closed, wakeful-resting experiments from 16 healthy volunteers after normal sleep and after PSD. We used independent component and cross-correlation analysis to study functional connectivity (fc), focusing on the DMN and ACN.
Results
After PSD, focal reductions of auto-correlation strength were detected in the posterior and anterior midline node of the DMN and in the lateral parietal and insular nodes of the ACN. Cross-correlation analysis confirmed reduced cortico-cortical connectivity within and between the DMN and ACN.
Conclusions
Increased sleep pressure is reflected in reduced fc of main DMN and ACN nodes during rest. Results have implications for understanding perceptual and cognitive changes after sleep deprivation and are relevant to clinical studies on conditions in which increased sleep propensity is present.
Similar content being viewed by others
References
Bruns A, Eckhorn R, Jokeit H, Ebner A (2000) Amplitude envelope correlation detects coupling among incoherent brain signals. Neuroreport 11(7): 1509–1514
Engel AK, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2(10): 704–716
Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A (2003) Spontaneously emerging cortical representations of visual attributes. Nature 425(6961): 954–956
Grinvald A, Arieli A, Tsodyks M, Kenet T (2003) Neuronal assemblies: single cortical neurons are obedient members of a huge orchestra. Biopolymers 68(3): 422–436
Leopold DA, Murayama Y, Logothetis NK (2003) Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex 13(4): 422–433
Buzsaki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304(5679): 1926–1929
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9): 700–711
Auer DP (2008) Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging 26(7): 1055– 1064
Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103(37): 13848–13853
Biswal BB, Van Kylen J, Hyde JS (1997) Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 10(4-5): 165–170
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98(2): 676–682
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102(27): 9673–9678
Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007) Wandering minds: the default network and stimulus-independent thought. Science 315(5810): 393–395
Cabeza R, Nyberg L (2000) Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12(1): 1–47
Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103(26): 10046–10051
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3(3): 201–215
Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH (2008) Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp 29(6): 671–682
Greicius MD, Menon V (2004) Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 16(9): 1484–1492
Greicius MD, Supekar K, Menon V, Dougherty RF (2009) Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19(1): 72–78
Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH (2009) Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci USA 106(27): 11376–11381
Sämann PG, Wehrle R, Spoormaker V, Hoehn D, Peters H, Holsboer F, Czisch M (2009) Development of the brain default mode network from wakefulness into slow wave sleep. Proceedings 17th scientific meeting, international society for magnetic resonance in medicine p 126
Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME (2009) Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA 106(11): 4489–4494
Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA (2008) Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18(8): 1856–1864
Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V (2008) Persistent default-mode network connectivity during light sedation. Hum Brain Mapp 29(7): 839–847
Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ (2009) Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 33(3): 279–296
Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM (2007) Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci USA 104(47): 18760–18765
Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101(13): 4637–4642
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62(5): 429–437
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME (2009) The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA 106(6): 1942–1947
Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S (2008) Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function?. Ann NY Acad Sci 1129: 119–129
Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S (2007) Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104(29): 12187–12192
Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK (2009) Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis 51(4): 294–302
Durmer JS, Dinges DF (2005) Neurocognitive consequences of sleep deprivation. Semin Neurol 25(1): 117–129
Tomasi D, Wang RL, Telang F, Boronikolas V, Jayne MC, Wang GJ, Fowler JS, Volkow ND (2009) Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex 19(1): 233–240
Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ (2003) Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12(1): 1–12
Giedke H, Schwarzler F (2002) Therapeutic use of sleep deprivation in depression. Sleep Med Rev 6(5): 361–377
Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB (2000) Altered brain response to verbal learning following sleep deprivation. Nature 403(6770): 655–657
Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC (1999) Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport 10(18): 3745–3748
Chee MW, Choo WC (2004) Functional imaging of working memory after 24 h of total sleep deprivation. J Neurosci 24(19): 4560–4567
Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MW (2005) Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage 25(2): 579–587
Mu Q, Mishory A, Johnson KA, Nahas Z, Kozel FA, Yamanaka K, Bohning DE, George MS (2005) Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep 28(4): 433–446
Drummond SP, Brown GG, Salamat JS, Gillin JC (2004) Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep 27(3): 445–451
Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG (2005) Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res 140(3): 211–223
Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ (2005) The neural basis of the psychomotor vigilance task. Sleep 28(9): 1059–1068
Ma L, Wang B, Chen X, Xiong J (2007) Detecting functional connectivity in the resting brain: a comparison between ICA and CCA. Magn Reson Imaging 25(1): 47–56
Wittchen HU, Lachner G, Wunderlich U, Pfister H (1998) Test-retest reliability of the computerized DSM-IV version of the Munich-Composite International Diagnostic Interview (M-CIDI). Soc Psychiatry Psychiatr Epidemiol 33(11): 568–578
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1): 97–113
Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2): 193–213
Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE (2004) Nonstationary cluster-size inference with random field and permutation methods. Neuroimage 22(2): 676–687
Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR, Iber C (2007) The visual scoring of sleep in adults. J Clin Sleep Med 3(2): 121–131
Strijkstra AM, Beersma DG, Drayer B, Halbesma N, Daan S (2003) Subjective sleepiness correlates negatively with global alpha (8–12 Hz) and positively with central frontal theta (4–8 Hz) frequencies in the human resting awake electroencephalogram. Neurosci Lett 340(1): 17–20
Finelli LA, Baumann H, Borbely AA, Achermann P (2000) Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience 101(3): 523–529
Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A (1995) Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep 18(10): 890–894
van den BJ, Neely G, Nilsson L, Knutsson A, Landstrom U (2005) Electroencephalography and subjective ratings of sleep deprivation. Sleep Med 6(3): 231–240
Vogt BA, Laureys S (2005) Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res 150: 205–217
Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D (2000) Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9(4): 335–352
Killgore WD (2007) Effects of sleep deprivation and morningness-eveningness traits on risk-taking. Psychol Rep 100(2): 613–626
Mayberg HS (2003) Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clin N Am 13(4): 805–815
Pizzagalli DA, Oakes TR, Davidson RJ (2003) Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology 40(6): 939–949
Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ (2001) Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 158(3): 405–415
Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009) The impact of global signal regression on resting state correlations: are anti-correlated networks introduced?. Neuroimage 44(3): 893–905
Fox MD, Zhang D, Snyder AZ, Raichle ME (2009) The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101(6): 3270–3283
Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F (2006) Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res Bull 70(4-6): 263–269
Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ (2002) Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience 114(4): 1047–1060
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sämann, P.G., Tully, C., Spoormaker, V.I. et al. Increased sleep pressure reduces resting state functional connectivity. Magn Reson Mater Phy 23, 375–389 (2010). https://doi.org/10.1007/s10334-010-0213-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-010-0213-z