Abstract
Background
Lipolysis-stimulated lipoprotein receptor (LSR), a lipid receptor, is associated with cancer progression. However, detailed effects on intracellular metabolism are unclear. We aimed to elucidate the mechanism of LSR-mediated lipid metabolism in gastric cancer.
Methods
We investigated lipid metabolic changes induced by lipoprotein administration in gastric cancer cells and evaluated the significance of LSR expression and lipid droplets formation in gastric cancer patients. The efficacy of inhibiting β-oxidation in gastric cancer cells was also examined in vitro and vivo.
Results
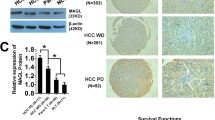
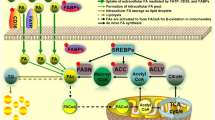
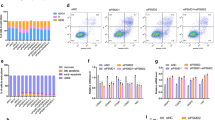
In gastric cancer cells, LSR promoted cellular uptake of lipoprotein and cell proliferation. Furthermore, the inhibition of LSR in gastric cancer cells expressing high levels of LSR counteracted both effects. Immunohistochemical analysis of human gastric cancer tissues showed that the increase in lipid droplets via LSR is a factor that influences prognosis. Lipidomics analysis of LSR-high-expressing gastric cancer cells revealed an increase in β-oxidation. Based on these results, we used etomoxir, a β-oxidation inhibitor, and found that it inhibited cell proliferation as well as the suppression of LSR. Similarly, in a mouse xenograft model of LSR-highly expressing gastric cancer cells, the tumor growth effect of high-fat diet feeding was counteracted by etomoxir, consistent with the Ki-67 labeling index.
Conclusions
We demonstrated that lipids are taken up into gastric cancer cells via LSR and cause an increase in β-oxidation, resulting in the promotion of cancer progression. Controlling LSR-mediated lipid metabolism may be a novel therapeutic strategy for gastric cancer.





Similar content being viewed by others
Data Availability
The datasets generated/analyzed during the current study are not publicly available because they contain private information pertaining to the research participants but are available on request from the corresponding author.
References
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Yanagimoto Y, Kurokawa Y, Doki Y. Essential updates 2021/2022: Perioperative and surgical treatments for gastric and esophagogastric junction cancer. Ann Gastroenterol Surg. 2023;7:698–708.
Yamashita K, Hosoda K, Niihara M, Hiki N. History and emerging trends in chemotherapy for gastric cancer. Ann Gastroenterol Surg. 2021;5:446–56.
Alsina M, Arrazubi V, Diez M, Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. 2023;20:155–70.
Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669–80.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Hsu PP, Sabatini DM. Cancer cell metabolism: warburg and beyond. Cell. 2008;134:703–7.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Schiliro C, Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 2021. https://doi.org/10.3390/cells10051056.
Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond). 2018;38:27.
Ohshima K, Morii E. Metabolic reprogramming of cancer cells during tumor progression and metastasis. Metabolites. 2021. https://doi.org/10.3390/metabo11010028.
Tan Y, Li J, Zhao G, Huang KC, Cardenas H, Wang Y, et al. Metabolic reprogramming from glycolysis to fatty acid uptake and beta-oxidation in platinum-resistant cancer cells. Nat Commun. 2022;13:4554.
Hiramatsu K, Serada S, Enomoto T, Takahashi Y, Nakagawa S, Nojima S, et al. LSR antibody therapy inhibits ovarian epithelial tumor growth by inhibiting lipid uptake. Cancer Res. 2018;78:516–27.
Sugase T, Takahashi T, Serada S, Fujimoto M, Ohkawara T, Hiramatsu K, et al. Lipolysis-stimulated lipoprotein receptor overexpression is a novel predictor of poor clinical prognosis and a potential therapeutic target in gastric cancer. Oncotarget. 2018;9:32917–28.
Nagase Y, Hiramatsu K, Funauchi M, Shiomi M, Masuda T, Kakuda M, et al. Anti-lipolysis-stimulated lipoprotein receptor monoclonal antibody as a novel therapeutic agent for endometrial cancer. BMC Cancer. 2022;22:679.
Yen FT, Masson M, Clossais-Besnard N, André P, Grosset JM, Bougueleret L, et al. Molecular cloning of a lipolysis-stimulated remnant receptor expressed in the liver. J Biol Chem. 1999;274:13390–8.
Bihain BE, Yen FT. The lipolysis stimulated receptor: a gene at last. Curr Opin Lipidol. 1998;9:221–4.
Sugase T, Takahashi T, Serada S, Nakatsuka R, Fujimoto M, Ohkawara T, et al. Suppressor of cytokine signaling-1 gene therapy induces potent antitumor effect in patient-derived esophageal squamous cell carcinoma xenograft mice. Int J Cancer. 2017;140:2608–21.
Sugase T, Takahashi T, Serada S, Fujimoto M, Hiramatsu K, Ohkawara T, et al. SOCS1 gene therapy improves radiosensitivity and enhances irradiation-induced DNA damage in esophageal squamous cell carcinoma. Cancer Res. 2017;77:6975–86.
Iwahashi N, Ikezaki M, Fujimoto M, Komohara Y, Fujiwara Y, Yamamoto M, et al. Lipid droplet accumulation independently predicts poor clinical prognosis in high-grade serous ovarian carcinoma. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13205251.
Fujimoto M, Matsuzaki I, Yamamoto Y, Yoshizawa A, Warigaya K, Iwahashi Y, et al. Adipophilin expression in cutaneous malignant melanoma. J Cutan Pathol. 2017;44:228–36.
Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue .IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13(1):27–9.
Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–5.
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503.
Judge A, Dodd MS. Metabolism. Essays Biochem. 2020;64:607–47.
Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20:137–55.
Jin Y, Tan Y, Wu J, Ren Z. Lipid droplets: a cellular organelle vital in cancer cells. Cell Death Discov. 2023;9:254.
García JM, Peña C, García V, Domínguez G, Muñoz C, Silva J, et al. Prognostic value of LISCH7 mRNA in plasma and tumor of colon cancer patients. Clin Cancer Res. 2007;13:6351–8.
Zhang M, Ma C. LSR promotes cell proliferation and invasion in lung cancer. Comput Math Methods Med. 2021;2021:6651907.
Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67:1493–504.
Okushin K, Tsutsumi T, Ikeuchi K, Kado A, Enooku K, Fujinaga H, et al. Heterozygous knockout of Bile salt export pump ameliorates liver steatosis in mice fed a high-fat diet. PLoS ONE. 2020;15: e0234750.
Dambrova M, Makrecka-Kuka M, Kuka J, Vilskersts R, Nordberg D, Attwood MM, et al. Acylcarnitines: nomenclature, biomarkers, therapeutic potential, drug targets, and clinical trials. Pharmacol Rev. 2022;74:506–51.
Oren Y, Tsabar M, Cuoco MS, Amir-Zilberstein L, Cabanos HF, Hütter JC, et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature. 2021;596:576–82.
Schlaepfer IR, Joshi M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology. 2020. https://doi.org/10.1210/endocr/bqz046.
Sawyer BT, Qamar L, Yamamoto TM, McMellen A, Watson ZL, Richer JK, et al. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol Cancer Res. 2020;18:1088–98.
Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond). 2000;99:27–35.
Hübinger A, Weikert G, Wolf HP, Gries FA. The effect of etomoxir on insulin sensitivity in type 2 diabetic patients. Horm Metab Res. 1992;24:115–8.
Hinderling VB, Schrauwen P, Langhans W, Westerterp-Plantenga MS. The effect of etomoxir on 24-h substrate oxidation and satiety in humans. Am J Clin Nutr. 2002;76:141–7.
Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond). 2007;113:205–12.
Yao CH, Liu GY, Wang R, Moon SH, Gross RW, Patti GJ. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. PLoS Biol. 2018;16: e2003782.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was partially funded by Research for Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (JP17im0210606 and JP20im0210111) for Tetsuji Naka.
Author information
Authors and Affiliations
Contributions
Kota Kawabata wrote the initial draft of the manuscript. Tsuyoshi Takahashi contributed to the analysis and interpretation of data and assisted in the preparation of the manuscript. All coauthors contributed to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to accuracy or integrity of any part of the work are appropriately investigated and resolved. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The Human Ethics Review Committee of Osaka University Graduate School of Medicine approved the protocol for this retrospective study and each participant provided written informed consent for study participation (ethical approval number: No. 23247).
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed. The Osaka University Animal Experiments Committee had given approval for the animal studies (ethical approval number: 04–108-000).
Consent for publication
Consent for publication was obtained from the person whose data were on the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawabata, K., Takahashi, T., Tanaka, K. et al. Lipolysis-stimulated lipoprotein receptor promote lipid uptake and fatty acid oxidation in gastric cancer. Gastric Cancer 27, 1258–1272 (2024). https://doi.org/10.1007/s10120-024-01552-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-024-01552-z