Abstract
Background
EGFR overexpression is a prognostic biomarker and is expected to be a predictive biomarker for anti-EGFR therapies in gastric cancer. However, few studies have reported the clinical impact of EGFR gene copy number (GCN) and its correlation with EGFR overexpression.
Methods
We used dual in situ hybridization (DISH) to detect EGFR GCN and chromosome 7 centromere (CEN7) in a set of tissue microarrays representing 855 patients with gastric cancer. These data were compared with those of immunohistochemical (IHC) analysis of EGFR expression to evaluate prognostic value.
Results
EGFR GCN gain (≥2.5 EGFR signals per cell) was detected in 194 patients (22.7 %) and indicated poor prognosis. Among 194 patients, EGFR amplification (EGFR/CEN7 ≥ 2.0) was observed in 29 patients (14.9 %), which was almost identical to the IHC 3+ subgroup and worst prognostic subgroup. Patients with EGFR GCN gain but not amplification, including those exhibiting polysomy, also exhibited poorer prognosis than GCN non-gain patients and were distributed between IHC 0/1+ and 2+ subgroups. GCN gain was frequently observed in patients with more advanced disease, but served as an independent prognostic factor regardless of the pathological stage.
Conclusions
EGFR GCN gain is a more accurate prognostic biomarker than EGFR overexpression in patients with gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second leading cause of cancer-related death worldwide. Annually, 989,600 new patients are diagnosed, and 738,000 people die of this disease [1]. Despite recent improvements in surgical approaches combined with chemotherapy and radiotherapy, the benefits of chemotherapy for advanced gastric cancer are still limited. Thus, there is an urgent need for the development of more effective drugs, particularly those targeting tumor progression mechanisms.
In gastric cancer, mutations in genes encoding components of the receptor tyrosine kinase (RTK)/RAS-signaling pathway are infrequent. In contrast, evidence indicates that amplification, overexpression [2–6], or epigenetic deregulation [7] may have an important role in tumor progression. The results of a recent ToGA trial revealed that the anti-HER2 antibody trastuzumab improves the survival of patients with HER2-positive advanced gastric cancer [2]. The results of other studies suggest the potential efficacy of targeted therapy against gene alterations in gastric cancers [8–10], and several other therapeutic drugs against these RTKs, including EGFR, are currently under investigation [11].
Deng et al. [5] detected amplification of genes encoding RTKs, including KRAS, HER2, EGFR, MET, and FGFR2, in approximately 37 % of gastric cancers, and suggested that amplification of these genes may act as an oncogenic driver often related to poor prognosis.
EGFR resides on chromosome 7p12 and encodes a 170-kDa RTK that contributes to cancer progression by mediating cellular proliferation, migration, invasion, and metastasis [12]. EGFR GCN alterations occur in a wide range of cancers and serve as a marker of poor prognosis. Inhibitors of EGFR activity include specific monoclonal antibodies and small molecule tyrosine kinase inhibitors that are used for standard therapy of lung and colorectal as well as head and neck cancers [13–16]. Therefore, EGFR is a strong candidate for molecular-targeted therapy in gastric cancer. EGFR overexpression and gene amplification serve as effective biomarkers for predicting the clinical benefit of anti-EGFR monoclonal antibodies for treating colorectal [17] and lung cancer [18]. The recent REAL3 (panitumumab) [19] and EXPAND (cetuximab) [20] randomized controlled phase III trials failed to demonstrate the efficacy of the anti-EGFR monoclonal antibodies for treating gastric cancer, presumably because precise patient inclusion criteria were not set for the trials. Several studies suggest that EGFR overexpression or gene amplification may serve as a potential biomarker for the efficacies of anti-EGFR therapies in gastric cancer [9, 21–23].
We reported that EGFR overexpression detected using immunohistochemistry (IHC) is associated with poor outcome of gastric cancer after curative resection [4]. However, the incidence of EGFR overexpression reported in previous studies varies from 2 % to 81 %, probably the result of differences in antibodies, scoring systems, and observers [4, 21, 24–32]. In contrast, the frequency of EGFR gene amplification in gastric cancers is consistently less variable (2–7 %), although most of these studies lacked a sufficiently large number of patients or a detailed EGFR GCN examination [3, 5, 22, 24, 26, 29, 30]. The correlation between overexpression and EGFR GCN has not been firmly established.
To address this issue, in the present study, we evaluated EGFR GCN in a large set of samples acquired from patients with gastric cancer and show that it correlates with protein expression and prognosis.
Materials and methods
Patients and pathological specimens
Formalin-fixed, paraffin-embedded (FFPE) gastric cancer tissues from consecutive patients who underwent surgical resection at the National Cancer Centre Hospital East in Japan from January 2003 to July 2007 were used. Construction of tissue microarrays (TMA) using these tumors is described elsewhere [33]. In brief, two separate tumor areas were selected based on depth and tumor histology, and a tissue core of 2 mm in diameter was obtained from each of the areas from each patient. Serial 4-µm sections of TMA blocks were analyzed using hematoxylin and eosin (H&E) staining, IHC, and dual in situ hybridization (DISH). Clinicopathological parameters including age, sex, tumor location, macroscopic type, histological type, lymphatic invasion, venous invasion, tumor depth (T), lymph node metastasis (N), HER2 status, resection margin, and adjuvant chemotherapy were acquired from medical records. Disease stage was classified using the TNM criteria of International Union Against Cancer (UICC), seventh edition. Overall survival was determined from the dates of surgical resection to death. The Institutional Review Board of the National Cancer Centre approved the study protocol.
EGFR immunohistochemistry
All reagents and instruments for IHC were manufactured by Ventana Medical Systems (Tucson, AZ, USA). FFPE sections were stained with Ventana Benchmark XT using a primary antibody against EGFR (clone 5B7) and Ventana iVIEW DAB Universal Kit, according to the manufacturer’s protocol. Stained slides were examined under light microscopy and were interpreted by E.H. and T.K. Membranous staining was scored (0, 1+, 2+, or 3+) as follows: 0 = undetectable membrane staining or <20 cancer cells with any intensity; 1+ = at least 20 cancer cells with faint membranous staining; 2+ = at least 20 cancer cells with weak to moderate membrane staining; and 3+ = at least 20 cancer cells with strong membrane staining. If the two cores from the same tumors had different IHC scores, the higher score was employed as the final IHC result.
Dual in situ hybridization (DISH) detection of EGFR and CEN7
All regents and instruments for DISH were manufactured by Ventana. DISH analyses for EGFR were carried out with Ventana Benchmark XT, using DNA cocktail probes [EGFR and chromosome 7 centromere (CEN7)] according to the manufacturer’s protocol. The number of EGFR signals (silver ISH detection, black dots) and CEN7 signals (red ISH detection, red dots) of 20 representative cells were counted in each core, and the higher score of two cores from each sample was employed as the final result. The EGFR/CEN7 ratio was calculated by dividing the total number of EGFR GCN signals by that of the CEN7 signals. Amplification of EGFR was defined as EGFR/CEN7 ≥ 2.0. Chromosome 7 polysomy was defined as the average of CEN7 signals ≥3.0 per cell. Cancer tissue cores that had no more than 1 signal for either EGFR or CEN7 per cell were excluded from further analyses.
Statistical analyses
For the multiple subgroup analyses of overall survival, the hazard ratio (HR) and 95 % confidence interval (CI) within each subgroup were summarized and displayed in a forest plot using the Cox regression model. An analysis of overall survival between subgroups was performed using the Kaplan–Meier method, and differences were compared using the log-rank test. The clinical characteristics of the two groups were compared using the chi-square test, Fisher’s exact test, and Cramér’s V. Univariate and multivariate analyses were conducted using the Cox regression model. Variables for multivariate analysis were selected by a backward stepwise approach using a significance level of P < 0.05 for variables remaining in the model. All reported P values are two sided, and the level of significance was P < 0.05. All statistical analyses were performed using IBM SPSS statistics version 18 for Windows (IBM, Armonk, NY, USA).
Results
Characteristics of patients and TMA specimens
After examining 2,012 cores from 1,006 patients using IHC and DISH, we excluded 151 patients from further analysis because no gastric cancer cells or deficient DISH signals were observed, leaving 855 patients for further analyses. The median (range) follow-up period of the surviving patients was 61.2 (1–121) months. The profile of clinicopathological treatment-related characteristics of these patients is shown in Online Resource 1.
EGFR expression and prognosis
IHC scores for the 855 patients were as follows: 463 (54.1 %) were IHC 0, 251 (29.3 %) were 1+, 114 (13.3 %) were 2+, and 27 (3.2 %) were 3+. The hazard ratios of IHC 1+, 2+, and 3+ subgroups compared with those of IHC 0 are described in Online Resource 2a. IHC 2+ and IHC 3+ results were significant indicators of poor prognosis, and IHC 3+ was identified as the strongest prognostic value in overall survival curve estimates (Online Resource 2b).
EGFR GCN and prognosis
The median copy numbers of EGFR and CEN7 per cell were 1.9 and 2.3, respectively, and the median EGFR/CEN7 ratio was 0.90. Chromosome 7 polysomy was observed in 154 patients (Online Resource 1). We divided the patients into four grades according to EGFR GCN per cell: grade 1 (GCN < 1.5), grade 2 (1.5 ≤ GCN < 2.0), grade 3 (2.0 ≤ GCN < 2.5), and grade 4 (2.5 ≤ GCN). The hazard ratio of each EGFR GCN subgroup for grade 1 is described in Fig. 1a. There were stepwise increases in the hazard ratio with increases in the GCN grade, and GCN grade 4 (HR = 2.51, P < 0.001) was the most informative prognostic factor.
Receiver operating characteristic (ROC) analysis was performed to define the optimal cutoff value that discriminates patients with poor prognosis (Fig. 1b). The ROC curve identified 2.35 signals of EGFR per cell as the optimal cutoff value discriminating between favorable and poor prognosis for 5-year survival. For further examination, we defined 2.5 EGFR signals per cell as the cutoff value for the definition of GCN gain, and 194 patients were assigned to this category.
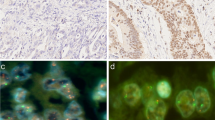
Among the 194 patients with GCN gain, the EGFR/CEN7 ratio was <2.0 for 165 patients, whereas EGFR was amplified in only 29. Among the 29 patients with EGFR amplification, 20 patients possessed >20 copies of EGFR per cell and formed a subgroup distinguished from the others (Fig. 2). Further, a clustered pattern of EGFR signals was observed in the nucleus for 27 of 29 patients with EGFR amplification (Fig. 3a). In the remaining 165 GCN patients without detectable gene amplification, chromosome 7 polysomy (Fig. 3b) was observed in 103 (53.1 %). The number of EGFR ISH signals without amplification or polysomy of the remaining 62 patients was slightly increased (Fig. 3c). The prognoses of patients with EGFR GCN gain were significantly worse compared with those without this gain. EGFR amplification was the strongest prognostic marker in the EGFR GCN subgroups (Fig. 4a). The prognostic value of EGFR GCN gain without amplification was still significant to predict poor clinical outcome from the absence of EGFR GCN gain.
Kaplan–Meier analysis of overall survival. a Comparison between patients without GCN gain (Non-gain, n = 661), with GCN gain but without amplification [Gain (non-amp), n = 165] and with both GCN gain and amplification [Gain (amp), n = 29] among 855 total patients. b Comparison between patients without GCN gain (Non-gain, n = 615) and with GCN gain (Gain, n = 99) among 714 patients with IHC scores of 0/1+. c Comparison between patients without GCN gain (Non-gain, n = 46) and with GCN gain (Gain, n = 68) among 114 patients with IHC scores of 2+. d Comparison between patients without heterogeneous GCN gain [Hetero GCN (−), n = 95] and with heterogeneous GCN gain [Hetero GCN (+), n = 70] among 165 patients with GCN gain but without amplification. e Comparison between patients without heterogeneous amplification [Hetero Amp (−), n = 5] and with heterogeneous amplification [Hetero Amp (+), n = 24] among 29 patients with amplification. f Comparison between patients with EGFR amplification observed in <50 % (n = 19), 50–80 % (n = 6), and ≥80 % (n = 4) of all tumor cells among 29 patients with amplification
Correlations between EGFR expression and EGFR GCN gain
EGFR was amplified in all 27 patients with IHC 3+, and GCN gain was detected in 59.6 % (68/114) of IHC 2+ patients (Table 1). GCN gain was a significant indicator of poor prognosis in the IHC 0/1+ (P = 0.014; Fig. 4b) and IHC 2+ subgroups (P = 0.042; Fig. 4c).
Correlation between clinicopathological characteristics and EGFR GCN gain
The correlations between clinicopathological characteristics and EGFR GCN gain are summarized in Table 2. EGFR GCN gain was more prevalent for patients with characteristics as follow: male, age >65 years, macroscopic type 2 or 3, differentiated type, presence of lymphatic or venous invasion, advanced tumor depth, lymph node metastasis, advanced stage, HER2-positive, R2 of resection margin, and adjuvant chemotherapy.
Multivariate analysis of prognostic factors for survival
Clinicopathological factors of 855 patients were analyzed using Cox regression to determine their prognostic significance. Thirteen factors were significant for overall survival as follows: age >65 years, macroscopic type 4, undifferentiated type, presence of lymphatic invasion, presence of venous invasion, T4 tumor depth, lymph node metastasis, TNM stage III/IV, R2 resection margin, IHC 2+, IHC 3+, EGFR GCN gain without amplification, and EGFR GCN gain with amplification (Table 3). The eight factors, which were identified using multivariate analysis of overall survival, correlated independently with worse prognosis as follows: age >65 years, macroscopic type 4, undifferentiated type, venous invasion, TNM stage III/IV, R2 of resection margin, EGFR GCN gain without amplification (HR = 1.53; 95 % CI = 1.11–2.10, P = 0.009), and EGFR GCN gain with amplification (HR = 2.46; 95 % CI = 1.44–4.21, P < 0.001) (Table 3). Note that IHC 2+ was not demonstrated as an independent indicator of poor prognosis. Further, IHC 3+ was excluded by multivariable analyses because of confounding GCN gain results with amplification. Even when survival analyses were adjusted for stage and resection margin, EGFR GCN gain was a significantly worse prognostic indicator in patients compared with the absence of GCN gain (Online Resource 3a/b).
Intratumoral EGFR GCN alteration heterogeneity
In the present study, a tumor was defined as heterogeneous if different EGFR GCN gain or amplification results were obtained from two separate TMA cores from the same tumor. Heterogeneity status of the EGFR GCN alteration was detected for 70 (42.4 %) of 165 patients with EGFR GCN gain and for 24 (82.8 %) of 29 patients with amplification. As shown in Fig. 4d, e, a heterogeneous EGFR GCN status tended to have better prognostic impact upon EGFR gene amplification as well as GCN gain tumors (P = 0.01 and P = 0.082, respectively). Among the 29 patients with amplification, the proportion of tumor cells exhibiting EGFR amplification in two TMA cores was <50 % in 19 patients (65.5 %), 50–80 % in 6 patients (20.7 %), and ≥80 % in 4 patients (13.8 %). The patients in whom ≥50 % of tumor cells exhibited EGFR amplification had a significantly poor prognosis (P = 0.006; Fig. 4f), and an increased proportion of cells exhibiting amplification was suggested to be associated with a poor prognosis.
Discussion
EGFR gene amplification results from an aberrant DNA replication and leads to a large number of gene copies integrated into either intrachromosomal tandem repeats or extrachromosomal double minutes and is a key mechanism of EGFR signaling activation [34]. In contrast, EGFR GCN gain is defined as GCN ≥ 2.5 per cell and results from various causes such as segmental chromosomal duplications or polysomy. EGFR GCN gain may accelerate EGFR-associated signaling, although the biological significance of EGFR GCN gain may differ from EGFR gene amplification in EGFR signaling activation.
We showed that EGFR amplification was a rare event (3.4 %) and indicated the worst prognostic impact. Although the criteria for EGFR amplification are not formally established and a wide range of EGFR/CEN7 ratios (1.7–6.0) were used in previous studies [3, 5, 22, 24, 26, 29, 30], the frequency of EGFR amplification did not vary among these studies, which may be attributed to the fact that the EGFR/CEN7 ratio is >10.0 in most patients with EGFR amplification, and a defined cutoff value would not influence the results (Fig. 2). For example, in the present study, 27 of 29 patients with EGFR amplification exhibited large clusters of signals in the nucleus.
Few studies have revealed a correlation between clinical outcome and EGFR GCN gain without amplification in gastric cancer. In the present study, we found that prognosis worsened as EGFR GCN increased. We defined the cutoff value of GCN gain as 2.5 EGFR signals per cell in the present study, and this value effectively delineated between poor and favorable prognoses. Thus, EGFR GCN gain without amplification was also a poor prognostic factor. The pathological characteristics of patients with EGFR GCN gain, including lymphatic invasion, venous invasion, tumor depth, and lymph node metastasis, were more severe and frequent in advanced stages, suggesting that EGFR GCN gain increases EGFR RTK signaling and contributes to tumor progression. Therefore, EGFR GCN gain may serve as a potential biomarker for guiding anti-EGFR therapies.
Evidence indicates that polysomy of chromosome 17 in patients with breast cancer may represent a clinical response to trastuzumab, even in patients without amplification of HER2 [35]. Polysomy of chromosome 17 indicates some degree of clinical benefit for the use of trastuzumab in gastric cancer as well [36]. Our data raise the possibility that chromosome 7 polysomy may represent a potential biomarker for EGFR therapy, although further studies are needed for corroboration.
No published data have been available to show a correlation between EGFR GCN and EGFR overexpression in patients with gastric cancer. Although there are no acknowledged consensus criteria for the histological determination of EGFR status in gastric cancer, some studies show that strong IHC staining of the tumor cell membrane (corresponding to IHC 3+ or IHC 2+/3+ in the present study) indicates poor prognosis [4, 26]. In the present study, we used a monoclonal anti-EGFR antibody (clone 5B7) raised against the functionally active intracellular domain of EGFR. When used for studies on colon cancer, this antibody exhibited higher sensitivity and specificity for EGFR overexpression than other anti-EGFR antibodies that bound to the external domain of the EGFR [37]. As expected, in the present study, EGFR amplification was detected in all IHC 3+ samples. The sensitivity and specificity of EGFR amplification corresponding to EGFR IHC 3+ were 100 % and 99.8 %, respectively. Moreover, we detected GCN gain in more than half the samples with IHC 2+, and this gain was a significant prognostic factor for the IHC 0/1+ and IHC 2+ subgroups. These results suggest that the evaluation of GCN gain is a more effective biomarker than the IHC score for identifying poor prognosis.
In the present study, we used the DISH assay to evaluate the EGFR and CEN7 copy numbers. Notably, in some patients, the number of EGFR signals per cell was less than 1, possibly because the sizes of the dots representing EGFR were occasionally smaller than those representing CEN7 and were thus more difficult to identify. Accordingly, the frequency of GCN gain observed in the present study was lower than the frequencies in previous reports that incorporated the fluorescence in situ hybridization (FISH) method [22]. Therefore, we employed a cutoff value of 2.5 EGFR signals per cell. Nevertheless, we assumed that evaluation of EGFR GCN status using DISH must have an advantage over evaluation using FISH, primarily because DISH particles can be evaluated via conventional light microscopy, facilitating the identification of tumor cells. In the present study, we showed that cells exhibiting EGFR amplification were distributed heterogeneously throughout tumors (82.8 %) and that an increased proportion of EGFR-amplified cells was associated with a poor prognosis. Therefore, it is important to examine the EGFR GCN status throughout the tumor area and to evaluate the proportion of tumor cells exhibiting EGFR amplification. DISH is recommended for HER2 testing by the ASCO/CAP 2013 guidelines for breast cancer [38] and proves advantageous because it enables examination of tumors exhibiting more heterogeneity, such as gastric cancer.
In conclusion, we showed here that EGFR amplification was relatively rare in gastric cancer, although EGFR GCN gain was observed in 22.7 % of the patients. EGFR GCN gain correlated significantly with the IHC results and may serve as a biomarker with increased sensitivity and specificity for predicting prognosis. EGFR GCN gain, especially if combined with amplification status and IHC score, may provide a superior method for distinguishing among patients who may benefit from therapies that target EGFR.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803–10.
Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000.
Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–84.
Matsumoto K, Arao T, Hamaguchi T, Shimada Y, Kato K, Oda I, et al. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br J Cancer. 2012;106:727–32.
Ushijima T, Hattori N. Molecular pathways: involvement of Helicobacter pylori-triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin Cancer Res. 2012;18:923–9.
Okamoto W, Okamoto I, Arao T, Kuwata K, Hatashita E, Yamaguchi H, et al. Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF-02341066) in gastric cancer positive for MET amplification. Mol Cancer Ther. 2012;11:1557–64.
Kim YH, Sasaki Y, Lee KH, Rha SY, Park S, Boku N, et al. Randomized phase II study of nimotuzumab, an anti-EGFR antibody, plus irinotecan in patients with 5-fluorouracil-based regimen-refractory advanced or recurrent gastric cancer in Korea and Japan: preliminary results. J Clin Oncol. 2011;29(suppl):87.
Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572–83.
Cidon EU, Ellis SG, Inam Y, Adeleke S, Zarif S, Geldart T. Molecular targeted agents for gastric cancer: a step forward towards personalized therapy. Cancers (Basel). 2013;5:64–91.
Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–63.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8.
Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31.
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–8.
Jiang Z, Li CX, Li FY, Wang XS. EGFR gene copy number as a prognostic marker in colorectal cancer patients treated with cetuximab or panitumumab: a systematic review and meta analysis. PLoS ONE 2013;8:e56205. doi:10.1371/journal.pone.0056205
Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42.
Waddell T, Chau I, Cunningham D, Gonzalez D, Frances A, Okines C, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–9.
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–9.
Han SW, Oh DY, Im SA, Park SR, Lee KW, Song HS, et al. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer. 2009;100:298–304.
Luber B, Deplazes J, Keller G, Walch A, Rauser S, Eichmann M, et al. Biomarker analysis of cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric and oesophago-gastric junction cancer: results from a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO). BMC Cancer. 2011;11:509.
Hotz B, Keilholz U, Fusi A, Buhr HJ, Hotz HG. In vitro and in vivo antitumor activity of cetuximab in human gastric cancer cell lines in relation to epidermal growth factor receptor (EGFR) expression and mutational phenotype. Gastric Cancer. 2012;15:252–64.
Kimura M, Tsuda H, Morita D, Ichikura T, Ogata S, Aida S, et al. A proposal for diagnostically meaningful criteria to classify increased epidermal growth factor receptor and c-erbB-2 gene copy numbers in gastric carcinoma, based on correlation of fluorescence in situ hybridization and immunohistochemical measurements. Virchows Arch. 2004;445:255–62.
Gamboa-Dominguez A, Dominguez-Fonseca C, Quintanilla-Martinez L, Reyes-Gutierrez E, Green D, Angeles-Angeles A, et al. Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients: a multivariate analysis using a standardized immunohistochemical detection system. Mod Pathol. 2004;17:579–87.
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology (Oxf). 2008;52:738–46.
Matsubara J, Yamada Y, Hirashima Y, Takahari D, Okita NT, Kato K, et al. Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clin Cancer Res. 2008;14:3022–9.
Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15:69–79.
Kim JS, Kim MA, Kim TM, Lee SH, Kim DW, Im SA, et al. Biomarker analysis in stage III–IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favourable survival. Br J Cancer. 2009;100:732–8.
Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, Da Costa WL Jr, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030–6.
Atmaca A, Werner D, Pauligk C, Steinmetz K, Wirtz R, Altmannsberger HM, et al. The prognostic impact of epidermal growth factor receptor in patients with metastatic gastric cancer. BMC Cancer. 2012;12:524.
Jacome AA, Wohnrath DR, Scapulatempo Neto C, Carneseca EC, Serrano SV, Viana LS, et al. Prognostic value of epidermal growth factor receptors in gastric cancer: a survival analysis by Weibull model incorporating long-term survivors. Gastric Cancer. 2014;17:76–86.
Aizawa M, Nagatsuma AK, Kitada K, Kuwata T, Fujii S, Kinoshita T, et al. Evaluation of HER2-based biology in 1,006 cases of gastric cancer in a Japanese population. Gastric Cancer. 2014;17:34–42.
Sesboue R, Le Pessot F, Di Fiore F, Frebourg T. EGFR alterations and response to anti-EGFR therapy: is it a matter of gene amplification or gene copy number gain? Br J Cancer. 2012;106:426–7 (author reply 428).
Hofmann M, Stoss O, Gaiser T, Kneitz H, Heinmoller P, Gutjahr T, et al. Central HER2 IHC and FISH analysis in a trastuzumab (Herceptin) phase II monotherapy study: assessment of test sensitivity and impact of chromosome 17 polysomy. J Clin Pathol. 2008;61:89–94.
Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol. 2013;31:4445–52.
Algars A, Lintunen M, Carpen O, Ristamaki R, Sundstrom J. EGFR gene copy number assessment from areas with highest EGFR expression predicts response to anti-EGFR therapy in colorectal cancer. Br J Cancer. 2011;105:255–62.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Acknowledgments
The authors thank Ms. Yuka Nakamura for her technical assistance and Ms. Erika Noebel for English language review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Higaki, E., Kuwata, T., Nagatsuma, A.K. et al. Gene copy number gain of EGFR is a poor prognostic biomarker in gastric cancer: evaluation of 855 patients with bright-field dual in situ hybridization (DISH) method. Gastric Cancer 19, 63–73 (2016). https://doi.org/10.1007/s10120-014-0449-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-014-0449-9