Abstract
The clinical presentation of SARS is nonspecific and diagnostic tests do not provide accurate results early in the disease course. Initial diagnosis remains reliant on clinical assessment. To identify features of the clinical assessment that are useful in SARS diagnosis, the exposure status and the prevalence and timing of symptoms, signs, laboratory and radiographic findings were determined for all adult patients admitted with suspected SARS during the Toronto SARS outbreak. Findings were compared between patients with laboratory-confirmed SARS and those in whom SARS was excluded by laboratory or public health investigation. Of 364 cases, 273 (75%) had confirmed SARS, 30 (8%) were excluded, and 61 (17%) remained indeterminate. Among confirmed cases, exposure occurred in the healthcare environment (80%) or in the households of affected patients (17%); community or travel-related cases were rare (<3%). Fever occurred in 97% of patients by the time of admission. Respiratory findings including cough, dyspnea and pulmonary infiltrates evolved later and were present in only 59, 37 and 68% of patients, respectively, at admission. Direct exposure, fever on the first day of illness, and elevated temperature, pulmonary infiltrates, lymphopenia and thrombocytopenia at admission were associated with confirmed cases. Rhinorrhea, sore throat, and an elevated neutrophil count at admission were associated with excluded cases. In the absence of fever or significant exposure, SARS is unlikely. Other clinical, laboratory and radiographic findings further raise or lower the likelihood of SARS and provide a rational basis for estimating the likelihood of SARS and directing initial management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the end of the global SARS outbreak, the World Health Organization has continued to recommend surveillance for the re-emergence of SARS, while acknowledging that prompt diagnosis remains a challenge due to the nonspecific nature of the clinical illness and the inability of diagnostic tests to provide sensitive and specific results early in the disease course [1]. Cases of SARS due to laboratory or animal exposure have been reported since the global outbreak ended, and these events highlight the importance of prompt recognition of cases in preventing larger outbreaks from occurring [1–3]. Toronto was the site of the largest SARS outbreak outside of Asia, but previous clinical descriptions of the Toronto outbreak did not include all patients and did not use microbiological methods to classify cases [4–8]. We conducted a retrospective study of the entire Toronto SARS outbreak, using microbiological methods for case classification, to identify epidemiological and clinical findings that may be useful in early diagnosis of SARS.
Materials and methods
Study design and study population
The charts of all adult (age ≥16 years) patients initially diagnosed with suspect or probable SARS based on World Health Organization or Health Canada criteria and admitted to a Toronto area hospital between February and June 2003 were reviewed. Patients were classified as confirmed SARS, indeterminate SARS or excluded SARS based on their exposure history, clinical illness and the results of microbiological testing. Patients were classified as confirmed SARS if they had a compatible clinical illness (fever or nonproductive cough or dyspnea), an exposure to SARS (direct contact with a known SARS case or travel to a SARS-endemic area or time spent at an institution where SARS transmission was occurring, within 12 days of symptom onset), and a positive microbiological test (positive acute or convalescent serology, or positive PCR from clinical or pathological specimens). SARS was excluded in patients that had negative convalescent serology, or when retrospective investigations ruled out an exposure to SARS (e.g. a case where the patient’s only exposure to SARS was direct contact with a suspected SARS patient who later was determined to be seronegative). Patients were classified as indeterminate if they had a documented exposure and compatible clinical history, but did not have definitive results from microbiological testing (i.e. convalescent serology was not performed, acute serology was not performed or was negative, and PCR testing was not performed or was negative).
Serological and microbiological methods
A description of the specimen collection protocol and the methods used for serologic and diagnostic testing has been published previously [9]. Serologic testing was performed at the National Microbiology Laboratory, Winnipeg, Manitoba and at Mount Sinai Hospital, Toronto, Ontario using an enzyme-linked immunosorbent assay and an immunofluorescent assay to detect SARS-CoV antibodies (EuroImmun, Lubeck, Germany). Serological testing was considered negative if a sample drawn 28 days or more after illness onset was negative and positive if IgM or IgG antibodies were detected at predefined dilutions [10]. RT-PCR was performed at the National Microbiology Laboratory, the Ontario Central Public Health Laboratory and at Mount Sinai Hospital.
Data collection
The hospital charts of all identified patients were reviewed by trained research nurses, and data was recorded on a standardized form. Ten percent of charts were randomly selected for duplicate abstraction. Data was double-entered into a computerized database. The study was approved by the research ethics boards at all participating hospitals and at McMaster University, Hamilton, Ontario.
Statistical analysis
Analysis was conducted using SAS version 8 (SAS institute, Cary, NC, USA). Variables were compared between groups using the chi-square or Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. To account for differences in illness duration at hospital admission, cases were stratified into quartile groups based on the time from symptom onset to admission. The Cochrane–Armitage trend test and Spearman’s correlation coefficient were used to test for trends in symptom incidence between quartile groups for categorical and continuous variables. Kaplan–Meier survival curves were used to plot time-to-event data.
Results
Classification of cases
For 367 adult patients admitted to hospital with possible SARS, 364 charts were available for review. SARS was excluded in 30 (8%) patients due to lack of a significant exposure (10), negative convalescent serology (17) or both (3). SARS was confirmed in 273 (75%) patients, based on positive serology (240), positive histopathology and PCR (10) or positive PCR alone (23). The remaining 61 (17%) cases were classified as indeterminate. A review of serological results from all inpatients tested during the outbreak did not identify any additional cases.
Demographics and exposures
For the confirmed cases, the median age was 45 years (interquartile range, 36–57 years), 63% were female and 83% had no comorbidities (Table 1). Exposure occurred in the healthcare environment in 80% of cases; in hospitals (76%), physician’s offices (2%) or during patient transport (2%). Transmission outside the healthcare environment occurred among the household contacts of cases (17%, n=44); travel-related disease was confirmed in two cases (excluding the index case), and community transmission resulted in eight confirmed cases, six of whom were exposed at the same community event.
Clinical presentation of confirmed cases
Because the onset of illness and the initial symptoms were difficult to discern in patients who developed SARS while hospitalized for another illness, these cases (n=15) were excluded from the analysis of clinical presentation. Results are for the confirmed cases, unless otherwise specified.
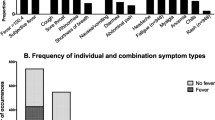
Patients were admitted to hospital a median of 4 days (interquartile range, 2–6 days) after illness onset. The initial symptoms of SARS among the confirmed cases, and the symptoms present at admission to hospital, are shown in Fig. 1. Initial symptoms included fever in 83%, nonspecific influenza-like symptoms such as myalgia, malaise or headache in 64%, respiratory symptoms in 31% and gastrointestinal symptoms such as diarrhea or nausea in 15%. At admission to hospital, fever was present in 93% of patients, influenza-like symptoms in 78%, respiratory symptoms in 67% (cough in 59%, dyspnea in 37%) and gastrointestinal symptoms in 32%. Following admission to hospital, fever resolved in a median of 3 days (interquartile range, 2–5 days), while respiratory symptoms continued to evolve. By the end of the first week in hospital, 88% of patients had respiratory symptoms (81% cough, 58% dyspnea).
Fever was the most common symptom, both initially and at admission. Of the 7% (n=17) of patients without fever at admission, eight had had fever that resolved prior to admission; thus, 97% of all patients had symptomatic fever at, or prior to, admission. Of the remaining nine patients, eight developed fever while in hospital, and one never had fever. SARS patients without fever by the time of admission were older (76 vs. 44 years, p=0.001), had more comorbid illness (67 vs. 13%, p<0.0001), and were more likely to die from SARS (44 vs. 7%, p=0.045) than patients with fever. Although symptomatic fever was present in 93% of patients on the day of admission, a documented temperature of ≥38.0°C was seen at admission in only 79% of cases.
Initial radiographic investigations identified pulmonary infiltrates in 68% (171/252) of patients, of whom 41% (70/171) had bilateral infiltrates. Infiltrates continued to evolve following admission and were present in 91% of patients by the end of the first week in hospital. Common laboratory abnormalities included leukopenia (leukocyte count <4.0×109/l) in 26%, lymphopenia (absolute lymphocyte count <1.0×109/l) in 57%, neutrophilia (absolute neutrophil count >7.5×109/l) in 9%, thrombocytopenia (platelet count <150×109/l) in 24%, elevated creatine kinase (CK >170 IU/l) in 30% and elevated lactate dehydrogenase (LDH >214 IU/l) in 50%.
The incidence of the clinical, radiographic and laboratory findings of SARS varied depending on the duration of illness at the time of presentation (Table 2). While fever and influenza-like symptoms were common throughout the first week of illness, respiratory symptoms and radiographic findings were significantly more common in patients presenting towards the end of the first week of illness: For example, the incidence of nonproductive cough, dyspnea or pulmonary infiltrates in patients presenting within 2 days of onset was 36, 24 and 48%, respectively; after 6 days of illness; these figures rose to 70, 53 and 78%, respectively. The time of onset of fever, dyspnea, nonproductive cough and pulmonary infiltrates relative to symptom onset are shown in Fig. 2. These results confirm that fever is an early symptom in most patients, while cough, dyspnea and infiltrates develop significantly later.
Comparison of the clinical and epidemiological features of patients with confirmed SARS and patients with excluded SARS identified several features that discriminate between these groups (Table 3). Findings associated with a confirmed diagnosis included direct exposure to a known case (OR, 2.34; 95%CI, 1.01–5.40), symptomatic fever as an initial symptom (OR, 5.07; 95%CI, 2.24–11.50), a documented temperature of 38.0°C on admission to hospital (OR, 2.6; 95%CI, 1.14–5.92), and the presence of a pulmonary infiltrate by the time of admission (OR, 2.46; 95%CI, 1.09–5.56). Findings associated with the absence of SARS included rhinorrhea initially (OR 0.14; 95% CI, 0.04–0.47) or at admission (OR 0.15; 95% CI, 0.05–0.50), sore throat initially (OR 0.23; 95% CI, 0.09–0.59), or at admission (OR 0.24; 95% CI, 0.10–0.59), vomiting at admission (OR 0.25; 95% CI, 0.08–0.77), or productive cough initially (OR 0.15; 95% CI, 0.02–0.92) or at admission (OR 0.23; 95% CI, 0.07–0.80).
When laboratory results were classified as normal or abnormal, a low leukocyte count (OR, 8.2; 95%CI, 1.09–62), low absolute lymphocyte count (OR, 5.01; 95%CI, 1.81–13.8), and low platelet count (OR, 6.8; 95%CI, 0.90–51.5) were predictive of confirmed SARS, while a high absolute neutrophil count (OR, 0.31; 95%CI, 0.11–0.85) made confirmed SARS less likely. Although the median CK was higher in patients with confirmed SARS (99 vs. 63 IU/l, p=0.04), the proportion of patients with CK above the normal range was not statistically significantly different between groups (31 vs. 16%, p=0.18). For LDH there was no difference in either the proportion of patients with elevated LDH between groups (50 vs. 41%, p=0.47) or in the median LDH (216 vs. 185 IU/l, p=0.10).
Discussion
This study provides the first description of the clinical presentation and early clinical course of SARS in all 258 laboratory-confirmed cases admitted to hospital during the Toronto SARS outbreak. Because evidence suggests that the sensitivity and specificity of the case definitions used for SARS diagnosis may be limited [11, 12] and because inclusion of even small numbers of non-SARS cases within a cohort of SARS patients can limit the ability to recognize features unique to SARS, we focused our analysis on the confirmed patients. Although it was not possible to determine the incidence of SARS in the group of 61 indeterminate patients, the incidence of clinical findings in this group tended to be intermediate between those with confirmed and those with excluded SARS, further suggesting that this was a mixed group of SARS and non-SARS cases.
Transmission of SARS in the Toronto outbreak was nosocomial in 80% of cases; an additional 17% of patients were exposed at home. Thus, 97% of cases had exposure either to the healthcare system or to an ill contact within the household (often a healthcare worker). Transmission due to travel or exposure within the community was distinctly uncommon. The tendency of SARS to transmit primarily within the nosocomial setting has been described in the majority of other SARS outbreaks [13–17].
The clinical features of SARS in confirmed patients demonstrated that SARS begins as a febrile illness, either with fever alone or with fever and a combination of nonspecific influenza-like symptoms such as myalgia and headache. Fever, although nonspecific, is the central symptom of SARS; it was often the initial symptom of illness, was present at or prior to admission in 97% of cases, and often persisted into the second week of illness. Respiratory symptoms, although a common feature of SARS frequently used in diagnostic algorithms, are often absent initially, rise in incidence over time, and are present in the majority of patients only late in the first week or early in the second week of illness, often after patients have been diagnosed and admitted to hospital. The incidence of pulmonary infiltrates seen on chest radiography parallels the evolution of respiratory symptoms, and many patients did not have documented infiltrates at the time of admission to hospital.
Although our comparative analysis of confirmed and excluded SARS cases did not identify any individual finding diagnostic of SARS, a number of features were identified that can increase or lower the probability of SARS. An approach that combines these findings with an understanding of the incidence and timing of the clinical, laboratory and radiographic findings of SARS will provide the best possible estimate of the likelihood of SARS in individual patients.
Our results suggest that, during an evolving SARS outbreak, and in the absence of evidence suggesting widespread transmission within the community, the absence of any direct or indirect exposure to the healthcare system makes a diagnosis of SARS unlikely, while direct exposure to a known case significantly raises the risk of SARS. Although the presence of fever is a nonspecific finding, the absence of a history of fever in a patient presenting to hospital several days after the onset of illness suggests that SARS is unlikely, given that 94% of SARS patients have fever by day 4 of illness, and 97% have fever by admission. On the other hand, the absence of respiratory symptoms or pulmonary infiltrates early in disease does not reduce the likelihood of SARS, as these may be late-onset findings. In some cases, careful follow-up with repeated clinical, laboratory and radiographic assessment can aid diagnosis, and this approach has been demonstrated to be useful [12, 18]. The presence of symptoms common in other infectious illnesses, but uncommon in SARS, is useful in lowering the probability of SARS, i.e., rhinorrhea, sore throat, productive cough and an elevated absolute neutrophil count are more common in patients without SARS. Features more common in confirmed SARS than in excluded cases included the presence of fever on the first day of illness, documented fever at admission, pulmonary infiltrates at admission, and leukopenia, lymphopenia or thrombocytopenia at admission. The presence of these findings may raise concerns about SARS, but they may be less useful diagnostically as they occur frequently in other conditions that may mimic SARS, such as community-acquired pneumonia [19]. Conversely, their absence lowers the likelihood of SARS, but none as much as the absence of fever at admission, given that the incidence of the other findings in confirmed SARS cases is considerably lower than the incidence of fever at admission.
Our study has several limitations. Data collection was retrospective and based on chart review. Biased or incomplete recording of clinical information could thus affect the accuracy of our results. Patients not admitted to the hospital were not considered. Patients that developed SARS following hospital admission were excluded from the analysis of clinical presentation. Finally, the number of patients in the excluded group was small, limiting our power to detect differences between the confirmed and excluded groups. Despite these limitations, we believe the results of our study are both valid and important. The unexpected emergence of SARS prevented large-scale prospective evaluations from being undertaken in most areas; we are therefore dependent on retrospective data to improve our understanding of SARS, despite the inherent limitations of this approach. Because asymptomatic SARS is rare, transmission has rarely been documented from mildly symptomatic cases, and >90% of seropositive patients identified in Toronto were admitted to hospital, we believe our results are widely applicable [20–22]. Because the 15 patients that developed SARS during hospitalization were excluded, extrapolation of our data to such patients should be made cautiously. Finally, although the small size of our excluded group suggests that the results of the between group comparisons should be interpreted cautiously, our results are consistent with those of other studies [23].
Ongoing surveillance for, and prompt diagnosis of, future SARS cases remains the most important strategy to prevent or limit future SARS outbreaks. Due to the limitations of current microbiological tests the best approach to diagnosis remains an assessment of exposure risk and clinical presentation. Our results identify a number of clinical, laboratory and radiographic findings that are useful in establishing the likelihood of SARS and demonstrate the importance of considering these factors in the context of disease duration. Taken together with the results of diagnostic testing for other pathogens and current global SARS epidemiology, these findings provide the best information upon which to establish a “pre-test” probability of SARS that can guide initial decisions pertaining to patient isolation, patient management and the ordering and interpretation of specific microbiological tests for SARS.
References
WHO (2004) WHO guidelines for the global surveillance of severe acute respiratory syndrome (SARS) http://www.who.int/csr/resources/publications/WHO_CDS_CSR_ARO_2004_1/en/index.html. Cited August 2005
Lim PL, Kurup A, Gopalakrishna G, Chan KP, Wong CW, Ng LC, Se-Thoe SY, Oon L, Bai X, Stanton LW, Ruan Y, Miller LD, Vega VB, James L, Ooi PL, Kai CS, Olsen SJ, Ang B, Leo YS (2004) Laboratory-acquired severe acute respiratory syndrome. N Engl J Med 350:1740–1745
Liang G, Chen Q, Xu J, Liu Y, Lim W, Peiris JS, Anderson LJ, Ruan L, Li H, Kan B, Di B, Cheng P, Chan KH, Erdman DD, Gu S, Yan X, Liang W, Zhou D, Haynes L, Duan S, Zhang X, Zheng H, Gao Y, Tong S, Li D, Fang L, Qin P, Xu W (2004) Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg Infect Dis 10:1774–1781
Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ (2003) Identification of severe acute respiratory syndrome in Canada. N Engl J Med 348:1995–2005
Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS (2003) Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289:2801–2809
Avendano M, Derkach P, Swan S (2003) Clinical course and management of SARS in health care workers in Toronto: a case series. CMAJ 168:1649–1660
Dwosh HA, Hong HH, Austgarden D, Herman S, Schabas R (2003) Identification and containment of an outbreak of SARS in a community hospital. CMAJ 168:1415–1420
Fowler RA, Lapinsky SE, Hallett D, Detsky AS, Sibbald WJ, Slutsky AS, Stewart TE (2003) Critically ill patients with severe acute respiratory syndrome. JAMA 290:367–373
Tang P, Louie M, Richardson SE, Smieja M, Simor AE, Jamieson F, Fearon M, Poutanen SM, Mazzulli T, Tellier R, Mahony J, Loeb M, Petrich A, Chernesky M, McGeer A, Low DE, Phillips E, Jones S, Bastien N, Li Y, Dick D, Grolla A, Fernando L, Booth TF, Henry B, Rachlis AR, Matukas LM, Rose DB, Lovinsky R, Walmsley S, Gold WL, Krajden S (2004) Interpretation of diagnostic laboratory tests for severe acute respiratory syndrome: the Toronto experience. CMAJ 170:47–54
Anonymous (2004) Updated interim surveillance case definition for severe acute respiratory syndrome (SARS)—United States, April 29, 2003. MMWR 52:391–393
Schrag SJ, Brooks JT, Van Beneden C, Parashar UD, Griffin PM, Anderson LJ, Bellini WJ, Benson RF, Erdman DD, Klimov A, Ksiazek TG, Peret TC, Talkington DF, Thacker WL, Tondella ML, Sampson JS, Hightower AW, Nordenberg DF, Plikaytis BD, Khan AS, Rosenstein NE, Treadwell TA, Whitney CG, Fiore AE, Durant TM, Perz JF, Wasley A, Feikin D, Herndon JL, Bower WA, Klibourn BW, Levy DA, Coronado VG, Buffington J, Dykewicz CA, Khabbaz RF, Chamberland ME (2003) SARS surveillance during emergency public health response, United States, March–July 2003. Emerg Infect Dis 10:185–194
Rainer TH, Cameron PA, Smit D, Ong KL, Hung AN, Nin DC, Ahuja AT, Si LC, Sung JJ (2003) Evaluation of WHO criteria for identifying patients with severe acute respiratory syndrome out of hospital: prospective observational study. BMJ 326:1354–1358
Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Abu-Raddad LJ, Ho LM, Thach TQ, Chau P, Chan KP, Lam TH, Tse LY, Tsang T, Liu SH, Kong JH, Lau EM, Ferguson NM, Anderson RM (2003) Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 361:1761–1766
Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348:1986–1994
Vu HT, Leitmeyer KC, Le DH, Miller MJ, Nguyen QH, Uyeki TM, Reynolds MG, Aagesen J, Nicholson KG, Vu QH, Bach HA, Plan AJ (2004) Clinical description of a completed outbreak of SARS in Vietnam, February–May 2003. Emerg Infect Dis 10:334–338
Wang JT, Sheng WH, Fang CT, Chen YC, Wang JL, Yu CJ, Chang SC, Yang PC (2004) Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis 10:818–824
Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, Kwan KM, Chan Y, Yim CF, Bek SL, Kor AC, Yap WS, Chelliah YR, Lai YC, Goh SK (2003) Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 290:374–380
Rainer TH, Chan PK, Ip M, Lee N, Hui DS, Smit D, Wu A, Ahuja AT, Tam JS, Sung JJ, Cameron P (2004) The spectrum of severe acute respiratory syndrome-associated coronavirus infection. Ann Intern Med 140:614–619
Muller MP, Tomlinson G, Marrie TJ, Tang P, McGeer A, Low DE, Detsky AS, Gold WL (2005) Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community-acquired pneumonia? Clin Infect Dis 40:1079–1086
Chen WQ, Lu CY, Wong TW, Ling WH, Lin ZN, Hao YT, Liu Q, Fang JQ, He Y, Luo FT, Jing J, Ling L, Ma X, Liu YM, Chen GH, Huang J, Jiang YS, Jiang WQ, Zou HQ, Yan GM (2005) Anti-SARS-CoV immunoglobulin G in healthcare workers, Guangzhou, China. Emerg Infect Dis 11:89–94
Ip M, Chan PK, Lee N, Wu A, Ng TK, Chan L, Ng A, Kwan HM, Tsang L, Chu I, Cheung JL, Sung JJ, Tam JS (2004) Seroprevalence of antibody to severe acute respiratory syndrome (SARS)-associated coronavirus among health care workers in SARS and non-SARS medical wards. Clin Infect Dis 38:e116–e118
Lee HK, Tso EY, Chau TN, Tsang OT, Choi KW, Lai TS (2003) Asymptomatic severe acute respiratory syndrome-associated coronavirus infection. Emerg Infect Dis 9:1491–1492
Leung GM, Rainer TH, Lau FL, Wong IO, Tong A, Wong TW, Kong JH, Hedley AJ, Lam TH (2004) A clinical prediction rule for diagnosing severe acute respiratory syndrome in the emergency department. Ann Intern Med 141:333–342
Acknowledgements
The authors would like to thank and acknowledge L. Moss, M. Keogh, J. Pound, M. Mione, J. DeJager and C. HarltonStezov and all others who assisted with data collection, data entry and data management. Thanks also to K. Austin for helpful comments on the manuscript. Finally, we would like to thank all Toronto area clinicians who assisted with case identification and who dedicated themselves to the care of these patients.
Members of the Canadian SARS Research Network.
Participant (Working Group): A. Ali, S. Asa, M. Avendano, M. Baqi, P. Derkash, A.S. Detsky, J. Downey, L. Dresser, I. Ephtimios, R. Fowler, M. Gershon, W. Gold, K. Gough, M. Herridge, R. Ilan, I. Kitai, S. Krajden, S. Lapinsky, M. Louie, M. Loutfy, R. Lovinsky, L. Matukas, B. Mederski, D.L. Moore, M.P. Muller, K. Ostrowska, N. Paul, E. Phillips, A. Rachlis, D. Rose, A. Sarabia, L. Sklar, R. Schabas, Z. Shainehouse, H. Shulman, M. Silverman, A. Simor, A. Slutsky, T. Stewart, R. Styra, C. Tansey, A. Turgay M. Varkul, S. Walmsley, P. Webster, B. Wolff, D. Yamamura (Clinical Working Group), G. Broukhanski, M. Chernesky, R. Devlin, M. Fearon, J. Fuller, F. Gharabaghi, I. Gugliemi, F. Jamieson, G. Johnson, A. Li, D. Low, L. Louie, A. Matlow, J. Mahoney, T. Mazzulli, A. Petrich, S. Poutanen, S. Richardson, M. Smieja, R. Skrastins, P. Tang, R. Tellier, M. Vearncombe, B. Willey, D. Zoutman (Diagnostics Group), B. Henry, A. McGeer, M. Ofner, S. Walter (Epidemiology Group), C. Bauch, J. Butany, D. Earn, D. Fisman, J. Ma (Modeling Group), J. Brunton, C. Cameron, M. Cameron, J. Coombs, A. Danesh, G. Downey, A. Edwards, R. Gorezynski, G. Levy, D. Kelvin, S. Keshavjee, G. Levy, P. Marsden, I. McGilvary, D. Persaud, J. Phillips, L. Ran, O. Rotstein, K. Seminovitch, R. Sekaly, J. Woodget, L. Zhang, L. Zu (Immunology Group), M. Loeb (Scientific Director).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Muller, M.P., Richardson, S.E., McGeer, A. et al. Early diagnosis of SARS: lessons from the Toronto SARS outbreak. Eur J Clin Microbiol Infect Dis 25, 230–237 (2006). https://doi.org/10.1007/s10096-006-0127-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-006-0127-x