Abstract
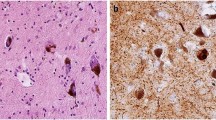
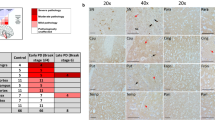
Alpha-synuclein, parkin, and synphilin-1 are proteins mainly involved in the pathogenesis of Lewy body (LB) diseases. mRNAs of all three undergo alternative splicing, so that the existence of various isoforms has been described. Since increasing evidence supports the importance of differential isoform-expression changes in disease development, we have established isoform-expression profiles in frontal cortices of LB disease brains in comparison with those of Alzheimer disease (AD) and control frontal cortices. The differential expression of four alpha-synuclein, seven parkin, and four synphilin-1 isoforms was ascertained by the use of isoform-specific primers and relative expression analysis with SybrGreen and beta-actin as an internal standard. The establishment of isoform-expression profiles revealed that these are disease specific. Moreover, isoform-expression deregulation of mainly one gene in each disease could be observed. All four alpha-synuclein isoforms were affected in the case of the pure form of dementia with LB, most parkin transcript variants in common LB disease, and all synphilin-1 isoforms in Parkinson disease. Only minor involvement was detected in AD. Finally, the existence of a proprietary isoform-expression profile in common LB disease indicates that this disease develops as a result of its own molecular mechanisms, and so, at the molecular level, it does not exactly share changes found in pure dementia with LB and AD. In conclusion, isoform-expression profiles in LB diseases represent additional evidence for the direct involvement of isoform-expression deregulation in the development of neurodegenerative disorders.


Similar content being viewed by others
References
Jellinger KA (2003) Neuropathological spectrum of synucleinopathies. Mov Disord 18(Suppl 6):S2–12
Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U (2002) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 249(Suppl 3):III/1–5
Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, Ben-Shlomo Y, Dickson DW, Lang AE, Chesselet MF, Langston WJ, Di Monte DA, Gasser T, Hagg T, Hardy J, Jenner P, Melamed E, Myers RH, Parker D Jr, Price DL (2007) The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J Neuropathol Exp Neurol 66:251–257
Litvan I, Chesselet MF, Gasser T, Di Monte DA, Parker D Jr, Hagg T, Hardy J, Jenner P, Myers RH, Price D, Hallett M, Langston WJ, Lang AE, Halliday G, Rocca W, Duyckaerts C, Dickson DW, Ben-Shlomo Y, Goetz CG, Melamed E (2007) The etiopathogenesis of Parkinson disease and suggestions for future research. Part II. J Neuropathol Exp Neurol 66:329–336
Neef D, Walling AD (2006) Dementia with Lewy bodies: an emerging disease. Am Fam Physician 73:1223–1229
McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, Carlos Machado J, O’Brien J, Playfer J, Reid W (2004) Dementia with Lewy bodies. Lancet Neurol 3:19–28
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A 95:6469–6473
Shults CW (2006) Lewy bodies. Proc Natl Acad Sci U S A 103:1661–1668
Beyer K, Ariza A (2007) Protein aggregation mechanisms in synucleinopathies: commonalities and differences. J Neuropathol Exp Neurol 66:965–974
Liani E, Eyal A, Avraham E, Shemer R, Szargel R, Berg D, Bornemann A, Riess O, Ross CA, Rott R, Engelender S (2004) Ubiquitylation of synphilin-1 and alpha-synuclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson’s disease. Proc Natl Acad Sci U S A 101:5500–5505
Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25:2002–2009
Tanji K, Tanaka T, Mori F, Kito K, Takahashi H, Wakabayashi K, Kamitani T (2006) NUB1 suppresses the formation of Lewy body-like inclusions by proteasomal degradation of synphilin-1. Am J Pathol 169:553–565
Wakabayashi K, Engelender S, Tanaka Y, Yoshimoto M, Mori F, Tsuji S, Ross CA, Takahashi H (2002) Immunocytochemical localization of synphilin-1, an alpha-synuclein-associated protein, in neurodegenerative disorders. Acta Neuropathol 103:209–214
Davidson WS, Jonas A, Clayton DF, George JM (1998) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273:9443–9449
Jao CC, Der-Sarkissian A, Chen J, Langen R (2004) Structure of membrane-bound alpha-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci U S A 101:8331–8336
Norris EH, Giasson BI, Lee VM (2004) Alpha-synuclein: normal function and role in neurodegenerative diseases. Curr Top Dev Biol 60:17–54
Vekrellis K, Rideout HJ, Stefanis L (2004) Neurobiology of alpha-synuclein. Mol Neurobiol 30:1–2119
Campion D, Martin C, Heilig R, Charbonnier F, Moreau V, Flaman JM, Petit JL, Hannequin D, Brice A, Frebourg T (1995) The NACP/synuclein gene: chromosomal assignment and screening for alterations in Alzheimer disease. Genomics 26:254–257
Ueda K, Saitoh T, Mori H (1994) Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer’s disease amyloid. Biochem Biophys Res Commun 205:1366–1372
Beyer K, Domingo-Sabat M, Lao JI, Carrato C, Ferrer I, Ariza A (2007) Identification and characterization of a new alpha-synuclein isoform and its role in Lewy body diseases. Neurogenetics 9:1364–6745
Beyer K, Humbert J, Ferrer A, Lao JI, Carrato C, López D, Ferrer I, Ariza A (2006) Low a-synuclein 126 mRNA levels in dementia with Lewy bodies and Alzheimer disease. NeuroReport 17:1327–1330
Beyer K, Lao JI, Carrato C, Mate JL, Lopez D, Ferrer I, Ariza A (2004) Differential expression of alpha-synuclein isoforms in dementia with Lewy bodies. Neuropathol Appl Neurobiol 30:601–607
Imai Y, Soda M, Takahashi R (2000) Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin–protein ligase activity. J Biol Chem 275:35661–35664
Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin–protein ligase. Nat Genet 25:302–305
Dagata V, Cavallaro S (2004) Parkin transcript variants in rat and human brain. Neurochem Res 29:1715–1724
Humbert J, Beyer K, Carrato C, Mate JL, Ferrer I, Ariza A (2007) Parkin and synphilin-1 isoform expression changes in Lewy body diseases. Neurobiol Dis 26:681–687
Ribeiro CS, Carneiro K, Ross CA, Menezes JR, Engelender S (2002) Synphilin-1 is developmentally localized to synaptic terminals, and its association with synaptic vesicles is modulated by alpha-synuclein. J Biol Chem 277:23927–23933
Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA (1999) Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet 22:110–114
Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM (2001) Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7:1144–1150
Eyal A, Szargel R, Avraham E, Liani E, Haskin J, Rott R, Engelender S (2006) Synphilin-1A: an aggregation-prone isoform of synphilin-1 that causes neuronal death and is present in aggregates from alpha-synucleinopathy patients. Proc Natl Acad Sci U S A 103:5917–5922
Preece P, Cairns NJ (2003) Quantifying mRNA in postmortem human brain: influence of gender, age at death, postmortem interval, brain pH, agonal state and inter-lobe mRNA variance. Brain Res Mol Brain Res 118:60–71
Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ (2003) An optimistic view for quantifying mRNA in post-mortem human brain. Brain Res Mol Brain Res 116:7–16
Karlen Y, McNair A, Perseguers S, Mazza C, Mermod N (2007) Statistical significance of quantitative PCR. BMC Bioinformatics 8:131
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64:1404–1410
McKeith IG (2006) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 9:417–423
Wolters E, Braak H (2006) Parkinson’s disease: premotor clinico-pathological correlations. J Neural Transm Suppl 70:309–319
Dalfo E, Ferrer I (2008) Early alpha-synuclein lipoxidation in neocortex in Lewy body diseases. Neurobiol Aging 29:408–417
Dalfo E, Portero-Otin M, Ayala V, Martinez A, Pamplona R, Ferrer I (2005) Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J Neuropathol Exp Neurol 64:816–830
Ferrer I, Perez E, Dalfó E, Barrachina M (2007) Abnormal levels of prohibitin and ATP synthase in the substantia nigra and frontal cortex in Parkinson’s disease. Neurosci Lett 415:205–209
Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25(8):2002–2009
Neystat M, Rzhetskaya M, Kholodilov N, Burke RE (2002) Analysis of synphilin-1 and synuclein interactions by yeast two-hybrid beta-galactosidase liquid assay. Neurosci Lett 325:119–123
Bandopadhyay R, Kingsbury AE, Muqit MM, Harvey K, Reid AR, Kilford L, Engelender S, Schlossmacher MG, Wood NW, Latchman DS, Harvey RJ, Lees AJ (2005) Synphilin-1 and parkin show overlapping expression patterns in human brain and form aggresomes in response to proteasomal inhibition. Neurobiol Dis 20:401–411
Eyal A, Engelender S (2006) Synphilin isoforms and the search for a cellular model of Lewy body formation in Parkinson’s disease. Cell Cycle 5:2082–2086
Huang Y, Cheung L, Rowe D, Halliday G (2004) Genetic contributions to Parkinson’s disease. Brain Res Brain Res Rev 46:44–70
Morris HR (2005) Genetics of Parkinson’s disease. Ann Med 37:86–96
Croisier E, Graeber MB (2006) Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol 112:517–530
Acknowledgments
We thank the University of Barcelona and Institute of Neuropathology Brain Banks, Barcelona, Spain, for their help with tissue procurement. We thank T. Jonahann for his editorial help. This work was supported by Spain’s Ministry of Health FIS grants PI 030132 and PI 050867, Catalonia’s AGAUR grant 2005SGR828, and MaratóTV3 grant 06/1410.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beyer, K., Domingo-Sàbat, M., Humbert, J. et al. Differential expression of alpha-synuclein, parkin, and synphilin-1 isoforms in Lewy body disease. Neurogenetics 9, 163–172 (2008). https://doi.org/10.1007/s10048-008-0124-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-008-0124-6