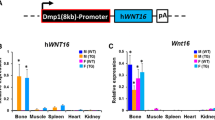
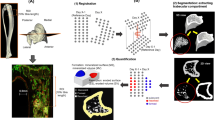
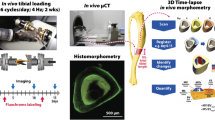
Abstract
Mechanical competence of bones is mainly associated with tissue quality that depends on proper bone metabolism processes. An imbalance in the regulation of bone metabolism leads to pathological changes in bone tissue leading to susceptibility to bone fractures and bone deterioration processes. Bone metabolism is regulated to a large extent by the members of the transforming growth factor-β superfamily, i.e., activins and bone morphogenetic proteins. However, their function is regulated by a single-chain protein called follistatin (FS). The aim of this study was to test the hypothesis that overexpression of FS in growing mice results in impairments in bone morphology and mechanical properties. Moreover, we wanted to investigate how geometrical, structural and material properties of bone tissue change with age. The experiment was performed on growing C57BL/6 TgNK14-mFst/6J mice, overexpressing FS (F mice) versus C57BL/6J mice used as controls (C mice). To establish how overexpression of FS influences bone tissue quality, we studied mice femurs to determine geometrical, structural and material properties of the skeleton. To determine mechanical resistance of bone tissue, femurs were loaded to failure in a three-point bending test. Obtained results indicated that overexpression of FS negatively influences bone metabolism. It was found that mutation results with a significant decrease of all measured biomechanical strength variables in F mice in comparison to C mice. Overexpression of FS leads to decreased quality of skeleton, increasing susceptibility to bone fractures.




Similar content being viewed by others
References
Bilezikjian LM, Leal AM, Blount AL, Corrigan AZ, Turnbull AV, Vale WW (2003) Rat anterior pituitary folliculostellate cells are target of interleukin-1β and major source of intrapituitary follistatin. Endocrinology 144:732–740
Bonadio J, Jepsen KJ, Mansoura MK, Jaenisch R, Kuhn JL, Goldstein SA (1993) A murine skeletal adaptation that significantly increases cortical bone mechanical properties. J Clin Invest 92:1697–1705
Brodt MD, Ellis CB, Silva MJ (1999) Growing C57B1/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res 14:2159–2166
Davison KS, Siminoski K, Adachi JD, Hanley DA, Goltzman D, Hodsman AB, Josse R, Kaiser S, Olszynski WP, Papaioannou A, Ste-Marie LG, Kendler DL, Tenenhouse A, Brown JP (2006) Bone strength: the whole is greater than the sum of its parts. Semin Arthritis Rheum 36:22–31
Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, van der Spek PJ, Visser JA, de Jongm FH, Pols HAP, van Leeuwen JP (2007) The activin A–follistatin system: potent regulator of human extracellular matrix mineralization. FASEB J 21:1–12
Ferretti JC, Capozza RF, Mondelo N, Zanchetta JR (1993) Interrelationships between densitometric, geometric and mechanical properties of rat femora: increases concerning mechanical regulation of bone modeling. J Bone Miner Res 8:1389–1395
Funaba M, Ogawa K, Murata T, Fujimura H, Murata E, Abe M, Takahashi M, Torii K (1996) Follistatin and activin in bone: expression and localization during endochondral bone development. Endocrinology 137:4250–4258
Isaksson H, Harjula T, Koistinen A, Ivarinen J, Seppänen K, Arokoski JPA, Brama PA, Jurvelin JS, Helminen HJ (2010) Collagen and mineral deposition of rabbit cortical bone during maturation and growth: effects on tissue properties. J Orthop Res 28:1626–1633
Jämsä T, Jalovaara P, Peng Z, Väänänen HK, Tuukkanen J (1998) Comparison of three-point bending test and peripheral quantitative computer tomography analysis in the evaluation of the strength of mouse femur and tibia. Bone 23:155–161
Kochanowska I, Chaberek S, Wojtowicz A, Marczyński B, Włodarski K, Dytko M, Ostrowski K (2007) Expression of genes for bone morphogenetic proteins BMP-2, BMP-4 and BMP-6 in various parts of the human skeleton. BMC Musculoskelet Disord 8:1–10
Kumar TR (2005) Too many follistatins: racing inside and getting out of the cell. Endocrinology 146:5048–5051
Lin S, Morrison J, Phillips D, Kretser D (2003) Regulation of ovarian function by the TGF-β superfamily and follistatin. Reproduction 126:133–148
Patel K (1998) Molecules in focus: follistatin. Int J Biochem Cell Biol 30:1087–1093
Phillips DJ, Kretser DM (1998) Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol 19:287–322
Piastowska-Ciesielska AW, Gralak MA (2010) Influence of a low dose of dietary soybean on bone properties and mineral status in young rats. BioFactors 36:451–458
Risbridger GP, Mellor SL, McPherson SJ, Schmitt JF (2001) The contribution of inhibins and activins to malignant prostate disease. Mol Cell Endocrinol 180:149–153
Saffar KPA, Rajaai NS (2009) How does the bone shaft geometry affect its bending properties. Am J Appl Sci 6:463–470
Schriefer JL, Robling AG, Warden SJ, Fournier AJ, Mason JJ, Turner CH (2005) A comparison of mechanical properties derived from multiple skeletal sites in mice. J Biomech 38:467–475
Schneyer AL, Wang Q, Sidis Y, Sluss PM (2004) Differential distribution of follistatin isoforms: application of a new FS315- specific immunoassay. J Clin Endocrinol Metab 89:5067–5075
Tardif G, Hum D, Pelletier JP, Boileau C, Ranger P, Martel-Pelleter J (2004) Differential gene expression and regulation of the bone morphogenetic protein antagonist follistatin and gremlin in normal and osteoartritic chondrocytes and synovial fibroblasts. Arthritis Rheum 50:2521–2530
Venken K, Callewaert F, Boonen S, Vanderschueren D (2008) Sex hormones, their receptors and bone health. Osteoporos Int 19:1517–1525
Vukicevic S, Sampath K (2004) Bone morphogenetic proteins: regeneration of bone and beyond. Birkhäuser, Basal
Wan M, Cao X (2005) BMP signaling in skeletal development. Biochem Biophys Res Commun 328:651–657
Wankell M, Munz B, Hübner G, Hans W, Wolf E, Goppelt A, Werner S (2001) Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J 20:5361–5372
Xiao YT, Xiang LX, Shao JZ (2007) Bone morphogenetic protein. Biochem Biophys Res Commun 362:550–553
Acknowledgments
This work was supported by a grant from the Ministry of Science and Higher Education No. NN401457838.
Conflict of interest
All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Gajos-Michniewicz, A., Pawlowska, E., Ochedalski, T. et al. The influence of follistatin on mechanical properties of bone tissue in growing mice with overexpression of follistatin. J Bone Miner Metab 30, 426–433 (2012). https://doi.org/10.1007/s00774-011-0347-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-011-0347-8