Abstract
The ATP-binding cassette transporter superfamily is present in all three domains of life. This ubiquitous class of integral membrane proteins have diverse biological functions, but their fundamental role involves the unidirectional translocation of compounds across cellular membranes in an ATP coupled process. The importance of this class of proteins in eukaryotic systems is well established as typified by their association with genetic diseases and roles in the multi-drug resistance of cancer. In stark contrast, the ABC transporters of prokaryotes have not been exhaustively investigated due to the sheer number of different roles and organisms in which they function. In this review, we examine the breadth of functions associated with microbial ABC transporters in the context of their contribution to bacterial pathogenicity and virulence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ATP-binding cassette (ABC) transporter superfamily is an ancient family of proteins with phylogenetic evidence indicating that significant functional diversification had already emerged prior to the evolutionary divergence of prokarya, archaea and eukarya (Davidson et al. 2008; Fuellen et al. 2005). The extensive functional flexibility of this family is such that evolution adopted these transporters to such an extent that ABC transporters are now present throughout all domains of life. Canonical ABC transporters are highly efficient translocation systems that couple the hydrolysis of ATP to the unidirectional movement of compounds across the phospholipid bilayer of cellular membranes. Two broad functional categories can be assigned to these transporters based on the vector of transport, ABC exporters and importers. ABC exporters are present in all known species and facilitate the translocation of compounds out of the cytoplasm of a cell. However, ABC importers, which are found in prokaryotes, archaea and more recently in plants (Shitan et al. 2003; Terasaka et al. 2005), drive the acquisition and transport of compounds from the extracellular milieu into the cytoplasm. In addition to these two main classes of transporters, the ABC superfamily also includes non-canonical energy-coupling factor importers and the non-transport systems that lack a transmembrane component, both of which are outside the scope of this review. For overviews of these systems, see Eitinger et al. (2011) and Hopfner and Tainer (2003), respectively.
ABC transporters share a common architecture
Despite the differing vectors of transport between the canonical ABC transporters, they have many conserved features. All canonical ABC transporters share a conserved core of four domains comprising two transmembrane domains (TMDs) and two nucleotide-binding domains (NBDs). These domains can be encoded in a variety of ways, generating components as single polypeptides to multi-domain fusions such as half-transporters encoded by TMD–NBD fusions (Fig. 1). The TMDs traverse the lipid bilayer in a series of α-helices, with the overall structure providing a channel through which compounds are transported. Attached to the cytosolic side of the TMDs are two NBDs that act as “motor” domains that hydrolyze ATP molecules to drive the overall translocation cycle in an energy-dependent manner. The compounds translocated by ABC transporters are often referred to as allocrites, as ATP is the classical substrate molecule that is hydrolyzed to the product ADP (Holland et al. 1990), whilst the allocrite is unchanged during transport.
Domain organization schematic of ABC transporters associated with bacterial pathogenicity. In Gram-negative bacteria, ABC transporters are localized to the cytoplasmic membrane (CM) and, in some circumstances, interact with other protein components embedded in the outer membrane (OM), the periplasm and CM. a The transmembrane domains (TMD—rectangles) and nucleotide binding domains (NBD—spheres/hexagons) for ABC exporters are shown for a homodimeric TMD–NBD fusion (i), a heterodimeric TMD–NBD fusion (ii), a homodimeric TMD–NBD Type I secretion system in complex with a membrane fusion protein (MFP) and outer membrane porin (OMP) (iii) and a heterooligomeric complex of two NBDs and two TMDs (iv). b A soluble substrate-binding protein (SBP—ovals), as would be found in the periplasmic compartment of Gram-negative bacterial pathogens, interacting with canonical ABC importers composed of a heterooligomeric complex of two NBDs and two TMDs (v), a heterooligomeric complex of two non-identical NBDs and two TMDs (vi), a heterooligomeric complex of two NBDs and two non-identical TMDs (vii), a heterooligomeric complex of two NBDs and a TMD fusion (viii) and a heteroligomeric complex of non-identical NBDs and TMDs (ix). Domains shaded in grey indicate encoding by a distinct polypeptide
The nucleotide binding domains
The NBDs are the conserved domain of the ABC transporter superfamily. They are often thought of as ‘motors’ attached to different TMDs to facilitate transport, although this is an oversimplification of the tight integration that couples these two domains. NBDs are typically between 200 and 300 amino acids in length and consist of a larger RecA-like catalytic domain and a smaller helical domain, the latter of which is unique to ABC transporters (for detailed reviews of NBD structure and function, see Davidson et al. (2008), Kerr (2002) and Moody and Thomas (2005)). The first structural information for the NBDs was obtained in 1998 (Hung et al. 1998). This work provided the foundation from which the dimerization of the NBDs in a ‘head-to-tail’ arrangement with motifs from each NBD contributing to the nucleotide binding sites was determined (Jones and George 1999). These early insights have been confirmed by numerous crystal structures of dimeric NBDs (Chen et al. 2003b; Hopfner et al. 2000) and NBDs associated with intact ABC transporters (Dawson and Locher 2006; Oldham et al. 2007; Pinkett et al. 2007).
Essential to the function of the NBDs are a number of conserved motifs that establish the interfacial contacts present in the NBD dimer, provide the nucleotide binding sites, facilitate ATP hydrolysis and couple thermodynamic work to the TMDs (for further details on motifs and their roles, see Davidson et al. (2008), Jones and George (2004), and Schneider and Hunke (1998)). The most important motifs for nucleotide binding are the P-loop and the ABC signature motif. The P-loop, also called the Walker A-motif, is located in the RecA-like subdomain and binds the β- and γ-phosphate groups of ADP or ATP. The ABC signature motif, which is also referred to as the LSGGQ motif (due to the consensus amino acid coding sequence), is located in the helical subdomain and contacts the nucleotide of the ATP-bound state. These motifs are arranged such that the P-loop from one monomer is positioned adjacent to the ABC signature sequence motif of the opposing monomer. Thus, the nucleotide-binding site is formed by the interaction of motifs between two NBD monomers. The presence of these motifs in each monomer of the NBD dimer thereby provides two ATP binding sites in the transporter. The catalytically functional conformation of the NBD dimer is induced by ATP binding. The consequence is a tight dimerization of two NBD monomers with ATP ‘sandwiched’ in between, hence the sandwich–dimer model presented in the literature (Smith et al. 2002). It is highly likely that ATP binds at both NBD binding sites as the hydrolysis of ATP has been observed to demonstrate strong positive cooperativity (Davidson et al. 1996; Greller et al. 1999; Liu et al. 1997; Zaitseva et al. 2005b).
In addition to the ATP-binding motifs, the Walker B and the H-loop motifs are present at the dimer–ATP-binding interface to assist in the hydrolysis of the bound nucleotide. The Walker B-motif is arranged as a β-strand in the RecA-like subdomain and coordinates a magnesium ion (Mg2+), via a water molecule essential for ATP hydrolysis. The H-loop, also referred to as the Switch motif, contains a conserved histidine residue that contacts the γ-phosphate of ATP and has a role in hydrolysis. Together these motifs facilitate the nucleophilic attack of a water molecule upon the γ-phosphate of the ATP molecule to release ADP and Pi (Hollenstein et al. 2007a; Zaitseva et al. 2005a). ATP hydrolysis only occurs in the tightly dimerized nucleotide-bound state of the NBDs. After ATP hydrolysis, the NBDs re-open to allow Pi to dissociate whilst the NBD dimer resides in a short-lived ADP-bound state until the nucleotide subsequently dissociates (Lu et al. 2005). Although nucleotide binding is a prerequisite for the formation of a catalytically functional NBD dimer, there are some notable exceptions in the nucleotide hydrolysis event. In some instances, one of the nucleotide-binding sites has a degenerate arrangement of motifs, rendering the site impaired or incapable of ATP hydrolysis, such as in the xenobiotic transporters LmrCD (Lubelski et al. 2006b) and TmrAB (Zutz et al. 2011). However, perturbation of the theoretically ‘optimal’ catalytic arrangement of the NBDs is not a widespread feature in prokaryotic ABC transporters. Although the functional basis for the distortion of a nucleotide-binding site, resulting in a non-equivalence of sites within an otherwise symmetrical transporter, is an issue of continuing research, the catalytic function of the NBDs is maintained by virtue of the remaining consensus ATP-binding site.
Additional motifs are also present in the NBDs to ensure efficient NBD–NBD interactions and interdomain coupling between the NBDs and the TMDs. The Q-loop, which is located in the loop region that connects the RecA-like and helical subdomains, has been shown to mediate interactions between the NBDs and TMDs (Dawson and Locher 2006; Hollenstein et al. 2007b; Pinkett et al. 2007). Located at the dimer interface as well as at the interface to the TMDs, this region is likely involved in interdomain signalling (Dawson et al. 2007). Another example is the D-loop, which has roles in NBD–NBD interactions and ensuring tight coupling of the ATP-binding sites in the NBDs to the allocrite binding sites within the TMDs. In addition to the motifs described above, some NBDs, notably those involved in import of compounds, also have accessory domains that may be involved in regulating the transport process (Biemans-Oldehinkel et al. 2006).
The transmembrane domains
The integral membrane components of the ABC transporters have relatively low sequence similarity and, accordingly, show greater diversity than the NBDs. ABC exporters have a conserved TMD core with each TMD containing six transmembrane (TM) helices, for a total of twelve helices. The crystal structures of prokaryotic ABC exporters indicate that the TMDs extend significantly beyond the boundary of the membrane into the cytoplasm. In the high-resolution structure of Sav1866 from Staphylococcus aureus, this extension was approximately 25 Å (Dawson and Locher 2006). Although lacking in a large cytoplasmic extension, ABC importers show considerable variety in the number and arrangement of their TM helices. This range spans from five TM helices per TMD, in the methionine importer MetI from Escherichia coli (Rees et al. 2009), to ten helices per TMD, in the vitamin B12 transporter BtuCD from E. coli (Locher et al. 2002). The number of helices is postulated to reflect the size of the channel required for allocrite transport by each ABC transporter, with larger compounds requiring transporters with larger channels. Allocrite translocation through the TMDs remains poorly understood and, until recently, it was thought that allocrites moved through the TMDs without directly interacting with them. However, recent studies on the E. coli maltose importer, MalFGK2A, indicated that this was not true for all ABC importers. In MalF, the TMD-encoding protein of the maltose transporter, an allocrite-binding site was clearly identified where one maltose molecule was bound by interactions with residues in the TMDs (Oldham et al. 2007). This suggests that, at least for some larger allocrites, there may be a necessary interaction between the TM helices and allocrites that occurs as part of the transport process.
The transport process involves the transmission of thermodynamic work from the NBDs to the TMDs. The coupling of this transmission involves a number of features, notably the Q-loop in the NBDs and the structurally conserved ‘coupling helices’ of the TMDs. The coupling helices show very little sequence conservation but are an architecturally conserved feature present in all ABC transporters. The coupling helices form the NBD–TMD interface (Locher 2009), where it is thought that nucleotide-induced conformational changes are transmitted via non-covalent interactions from the Q-loop region of the NBDs.
ABC exporters associated with bacterial virulence
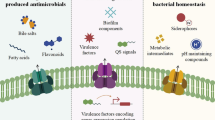
ABC exporters are the ubiquitous subfamily of ABC transporters present throughout the three domains of life. They are, in contrast to eukaryotes and prokaryotes, not as highly represented amongst archaea (Davidson et al. 2008). Numerous studies have presented classification schemes for this broad superfamily (Dassa and Bouige 2001; Davidson et al. 2008; Eitinger et al. 2011). The most recent classification scheme placed the majority of ABC exporters in the Class 1 ABC transporter family. For an excellent overview of the bacterial ABC transporter superfamily see Davidson et al. 2008. In this review, we have focussed on virulence-associated ABC transporters (Fig. 2). ABC transporters with an apparent efflux function exist in both Class 1 and Class 3 according to this scheme. Based on the transporter classification (TC) database (http://www.tcdb.org) (Saier et al. 2006), there are 40 subfamilies of prokaryotic ABC exporters, annotated with the TC identification (TCID) as the 3.A.1. family, that have been identified to date. These can be broadly subdivided into transporters associated with glycoconjugate biosynthesis, Type I protein secretion or xenobiotic efflux. The prokaryotic xenobiotic transporters are highly represented in recent literature because of their homology to eukaryotic transporters, which are predominantly Class 1 ABC transporters. However, the other transporters also play important roles in bacterial physiology where they are intimately involved in biosynthetic pathways, such as in glycan export (Cuthbertson et al. 2009), and in protein secretion, such as that of surface (S)-layer proteins, toxins and competence factors (Otto and Gotz 2001; Zaitseva et al. 2005a). In this section, we will examine the contribution of ABC exporters to the virulence of bacterial pathogens in mammalian hosts.
Roles associated with ABC transporters involved in bacterial pathogenicity in a model Gram-negative cell. ABC exporters include (a) Type I secretions systems associated with toxin, S-layer protein, siderophore, hydrolytic enzyme or antimicrobial peptide secretion, which have roles in adhesin and colonization of the host; (b) glycoconjugate and polysaccharide biogenesis pathways, which are involved in membrane biogenesis and immune evasion; and (c) xenobiotic efflux pathways involved in host environment tolerance and with postulated roles in antibiotic efflux. ABC importers (d) are associated with processes such as nutrient acquisition (e.g. metal ions, amino acids, vitamins and oligopeptides) and osmoprotection, which have crucial roles for successful colonization and promulgation in the host environment
Glycoconjugate biosynthesis
ABC transporters have a major role in prokaryotic glycoconjugate biosynthesis with four glycoconjugate-annotated transporter subfamilies with TC identifications (TCID) 3.A.1.101–4. Prokaryotes have up to three cell surface glycoconjugate biosynthetic pathways, which are referred to as the Wzx/Wzy-dependent, ABC transporter-dependent and synthase-dependent pathways, based on their inner membrane export mechanism. Importantly, these three general assembly export strategies are not confined to the assembly of any one class of glycoconjugate. The ABC transporter-dependent pathway mediates the translocation of glycans from all major classes of cell surface glycoconjugates including glycoproteins, teichoic acids, capsular polysaccharides (CPS), exopolysaccharide (EPS) and the O-antigen polysaccharide portion of lipopolysaccharide (LPS). For an excellent review on the role of ABC transporters in the broader aspects of glycoconjugate biosynthesis, see Cuthbertson et al. (2010).
All polysaccharides assembled by ABC transporters are fully polymerized by sequential glycosyl transfer that occurs at the cytoplasmic face of the inner membrane prior to translocation. The ABC transporter then exports the completed glycan across the inner membrane in an ATP-dependent manner. Further export to the outer membrane requires additional transport components such as a polysaccharide co-polymerase protein and an outer membrane polysaccharide export protein (for detailed reviews, see Cuthbertson et al. (2009) and Whitfield (2006)). The involvement of ABC transporters in glycan export was first recognized in studies of CPS biosynthesis in E. coli (Pavelka et al. 1991; Smith et al. 1990) and Haemophilus influenzae (Kroll et al. 1990), where loss of the ABC exporter resulted in the accumulation of the synthesized polymer in the cytoplasm. Since then, the dependence of other glycan biosynthetic pathways on ABC transporters has also been reported (Alaimo et al. 2006; Lazarevic and Karamata 1995; Zhang et al. 1993). Polysaccharides such as O-antigen, teichoic acids, CPS and EPS all have important roles in the virulence of pathogenic bacteria, where they fulfil a diverse range of roles including cell signalling, biofilm formation and evasion of the host immune responses.
Lipopolysaccharide biogenesis
The outer membrane of many Gram-negative bacteria is densely coated with LPS, a polysaccharide consisting of lipid A and a core oligosaccharide (the complex of which we will refer to as the lipid A core) and a repeated O-antigen polysaccharide tail. LPS biogenesis utilizes ABC transporters for translocation of both of these components. The O-antigen of LPS is one of the most variable Gram-negative cell constituents and a major contributor to the antigenic variability of the cell surface. The O-antigen consists of repeating oligosaccharide units (O-units) of two to eight different sugar residues. Antigenic variation arises from the different sugar moieties and the arrangements and linkages within and between the O-units. The O-antigen is presented on the cell surface and, due to its high immunogenicity, undergoes intense selection pressure from environmental factors and the host immune response. Consequently, this has led to the maintenance of diverse O-antigen isoforms within individual species, such as E. coli (Kauffmann 1947), and forms the basis of serotyping classifications for Gram-negative bacteria. The ABC transporter Wzt/Wzm, which has a respective TMD/NBD architecture, facilitates O-antigen biogenesis in a number of pathogenic and non-pathogenic bacterial species (Cuthbertson et al. 2010; Whitfield 2006). This pathway is not essential for cell viability, but the loss of the export pathway does result in cytotoxic accumulation of polymer unless the stress is relieved by other mutations (Clarke et al. 2004; Cuthbertson et al. 2005). The O-antigen is a major virulence factor in the host–pathogen interaction, such as in pathogenic E. coli and Klebsiella pneumoniae, where it protects the invading bacterium from killing by membrane-directed compounds, such as antimicrobial peptides, and aids in the evasion of complement-mediated killing and bacterial colonization (Achtman and Pluschke 1986; Bengoechea et al. 2004; Pluschke et al. 1983a, b; Whitfield et al. 1991). Mutagenesis studies in pathogenic E. coli have also shown that loss of O-antigen reduces survival and decreases the virulence of the bacterium (Achtman and Pluschke 1986; Pluschke et al. 1983a, b).
The other constituent of LPS, the lipid A core, is transported across the inner membrane by the ABC transporter MsbA (Raetz et al. 2007). Although classified in the 3.A.1.106.1 group, its physiological role is in LPS biosynthesis and so it will be addressed in this section. The lipid A anchors LPS to the outer membrane and is covalently attached to the O-antigen via its core oligosaccharide domain. Lipid A is highly immunogenic and is recognized by the innate immune system by cell surface receptors including Toll-like receptor 4, MD-2 and CD14 (Beutler and Poltorak 2000; Park et al. 2009; Poltorak et al. 1998). MsbA has also been proposed to be a general phospholipid flippase, but there remains a paucity of definitive biochemical evidence to support this interpretation. The possible involvement of MsbA in phospholipid export has arisen from the observation that loss of MsbA in Gram-negative bacteria, notably E. coli and Pseudomonas aeruginosa, was lethal (Doerrler et al. 2004, 2001; Ghanei et al. 2007). However, in Neisseria meningitidis, loss of MsbA did not affect viability but instead correlated with a very low LPS content (Steeghs et al. 1998). Thus, it has since been proposed that the effects observed on phospholipid transport, in msbA conditional mutant strains, may have been a secondary effect due to accumulation of toxic concentrations of lipid A precursors in the cytoplasm (Tefsen et al. 2005). An intriguing facet of MsbA function observed in in vitro studies is its capacity for xenobiotic binding and efflux (Reuter et al. 2003; Siarheyeva and Sharom 2009). However, the significance of this xenobiotic efflux capability in the context of bacterial physiology remains to be elucidated.
Teichoic acid
In Gram-positive pathogens peptidoglycan (PG) is the most conserved component of the cell envelope and is densely populated with anionic polymers of teichoic acids. These polymers, which can comprise nearly 50% of the cell wall mass, are translocated to the cell surface via an ABC transporter where they are covalently attached to PG (Lazarevic and Karamata 1995). The ABC transporters responsible for teichoic acid translocation, TagGH in Bacillus subtilis (Lazarevic and Karamata 1995) and TarGH in Staphylococcus aureus (Schirner et al. 2011), have been shown to be essential for bacterial viability. Teichoic acids are a virulence factor in several bacterial pathogens where they have been shown to function as non-proteinaceous adhesins. In Sta. aureus, teichoic acids play an important role in the colonization of both abiotic (Gotz 2002) and biotic surfaces, most notably in the adherence to human epithelial and endothelial cells during host colonization (Weidenmaier et al. 2005). In the opportunistic pathogen Sta. epidermidis, teichoic acid has been shown to be involved in adherence to fibronectin (Hussain et al. 2001). However, the teichoic acid secretion system of Sta. epidermidis has not yet been defined.
Capsular and exopolysaccharides
Secreted prokaryotic polysaccharides include the tightly attached CPS, which form a discrete surface layer also known as the capsule, and the secreted EPS, which only retains a limited association with the cell surface. CPS and EPS are large hydrophilic polymers with masses in the range of 105 to 106 Da whose challenging biosynthesis involves a complex inner membrane assembly process prior to translocation across the periplasm and the outer membrane. In E. coli, CPS is translocated across the inner membrane by ABC transporter KpsMT where KpsM is the TMD and KpsT is the NBD (Kroncke et al. 1990a, b; Pavelka et al. 1994, 1991; Pigeon and Silver 1994). Polymer translocation across the periplasm and the outer membrane requires KpsE, a polysaccharide copolymerase, and KpsD, an outer membrane export protein (Arrecubieta et al. 2001; Bronner et al. 1993; McNulty et al. 2006; Pazzani et al. 1993; Silver et al. 1987). These proteins are proposed to interact, generating a structure functionally analogous to a tripartite drug efflux pump (Higgins et al. 2004). CPS are also known to act as virulence determinants in pathogenic bacteria where they have roles in resisting complement-mediated killing, preventing adhesion of monocytes and neutrophils during host invasion, and in resisting cationic peptides. The role of secreted CPS in virulence has been observed in N. meningitidis (Frosch et al. 1991), H. influenzae (Kroll et al. 1990; Moxon and Vaughn 1981), Campylobacter jejuni (Keo et al. 2011) and Salmonella enterica serovar Typhimurium (Hashimoto et al. 1993).
Secreted EPS is also known to be an important virulence determinant in many pathogenic bacteria. In Brucella abortus, a facultative intracellular bacterial pathogen which causes abortion in domestic animals and undulant fever in humans, the ABC transporter ExsA was identified as a putative extracellular polysaccharide exporter (Rosinha et al. 2002). ExsA was first identified in Rhizobium meliloti where the ExsA gene was responsible for the secretion of the polysaccharide succinoglycan (Becker et al. 1995). Although the virulence mechanisms of Brucella spp. are not fully understood, decreased survival of the ΔexsA mutant Brucella in mice compared with the survival of the parental strain demonstrated that ExsA was required for full virulence (Rosinha et al. 2002).
Protein secretion
Prokaryotic protein secretion predominantly occurs via the Type II secretory pathway but a number of additional translocation systems, referred to as Types I and III through VI, also provide important protein secretory pathways (for reviews, see Fronzes et al. (2009) and Saier et al. (2008)). Notably, the Type I secretory pathway is an ABC transporter-dependent mechanism. To date, more than 25 families of ABC exporters (TCIDs: 3.A.1.109–113, 116, 118, 119, 123, 124, 126–134) have been associated with Type I protein secretion functionality. These families translocate a broad range of exoproteins including toxins, antimicrobial peptides, siderophores, hydrolytic enzymes and S-layer proteins. Many of the secreted proteins have roles in facilitating invasion or survival in the host, while the antimicrobial peptides have roles in competition against other bacteria but are not directly implicated in their virulence.
Type I secretion systems have been studied in detail in Gram-negative bacteria where they operate in a continuous process to translocate proteins across both the inner and the outer membrane (Zaitseva et al. 2005a). The Type I translocon, which facilitates multi-membrane translocation, is a complex of three proteins that includes an ABC transporter, a membrane-fusion protein (MFP) and an outer membrane protein (OMP). The ABC transporter recognizes the target protein and hydrolyzes ATP to drive protein translocation. The MFP is a periplasmic spanning protein, which has one transmembrane segment anchored in the inner membrane and connects the complex to the OMP during formation of the translocon (Schulein et al. 1992). The OMP is an outer membrane porin protein, such as TolC, that has an extended periplasmic domain and forms the tunnel across the outer membrane (Koronakis et al. 2000). The majority of Type I secreted proteins of Gram-negative bacteria feature a ~60 amino acid carboxyl terminal secretion sequence, which is not removed during translocation and is recognized by the ABC transporter. The secretion process is assumed to occur in a post-translational manner, with the allocrite protein in a largely unfolded conformation (Bakkes et al. 2010; Zaitseva et al. 2005a).
Toxins
The best-characterized Type I secretion systems belong to the repeats-in-toxin (RTX) exoprotein family. Their name is derived from the repeated glycine- and aspartate-rich sequences located in the carboxy-terminal region of the protein. These repeats form binding sites for calcium (Ca2+) ions and adopt a β-roll structure upon ion binding in the extracellular space (Baumann et al. 1993). Members of this protein family exhibit a broad range of sizes (from 40 to >600 kDa) and biological activities, and although the nomenclature of this family is synonymous with the pore-forming cytotoxins, a number of other secreted proteins, proteases and lipases also belong to the RTX family (for a detailed review, see Linhartova et al. (2010)). These other exoproteins can also be significant virulence factors in mammalian diseases.
RTX toxins are Type I-dependent secreted proteins that are present in a broad range of Gram-negative mammalian pathogens such as HlyA and EhxA from E. coli (Goebel and Hedgpeth 1982; Schmidt et al. 1995), CyaA from Bordetella pertussis (Glaser et al. 1988), PvxA from Proteus vulgaris (Welch et al. 1981), ApxIA, ApxIIA, ApxIIIA and ApxIVA from Actinobacillus pleuropneumoniae (Chang et al. 1989; Frey et al. 1991; Jansen et al. 1993; Schaller et al. 1999), VcRtxA or MARTX VC from Vibrio cholerae (Lin et al. 1999), MbxA from Moraxella bovis (Angelos et al. 2003) and MmxA from Mannheimia varigena (Chang et al. 1993). The best-characterized Type I secretion system is the RTX α-hemolysin (Hly) secretion system of E. coli. It is commonly found in uropathogenic E. coli (Welch et al. 1981) where it is an important virulence factor due to its cytolytic activity against a broad range of mammalian cell types (e.g. erythrocytes, granulocytes, monocytes, endothelial cells and renal epithelial cells from mice, ruminants and primates). The hlyCABD operon encodes the genes required for the Type I secretion system and the synthesis of HlyA (Hess et al. 1986). The location of this operon, either on chromosome-embedded pathogenicity islands or on transmissible plasmids, suggests possible transmission by horizontal gene transfer between Gram-negative bacteria (Knapp et al. 1986; Ludwig and Goebel 1999).
The Hly translocon consists of the ABC transporter HlyB (Wang et al. 1991), the MFP HlyD (Johnson and Church 1999; Schulein et al. 1992) and the OMP TolC (Koronakis et al. 2000). HlyC is an acyl transferase involved in the maturation of HlyA. HlyA is a 107-kDa cytolysin and is thought to be largely unfolded in the cytoplasm due to the tightly restricted availability of Ca2+ ions. The interaction of the allocrite HlyA with HlyB and HlyD triggers recruitment of TolC and creates a transient channel–tunnel complex from the bacterial cytosol to the extracellular milieu (Wickner and Schekman 2005; Zaitseva et al. 2005a). Assembly of the Hly translocon occurs independent of nucleotide binding, but ATP hydrolysis by HlyB is required for HlyA export (Thanabalu et al. 1998). Once exported, HlyA folds in the extracellular space to form the biologically active toxin.
HlyA mediates its cytolytic activity by inserting asymmetrically into the outer leaflet of eukaryotic plasma membranes in which it generates hydrophilic transmembrane pores (Menestrina et al. 1995a, b). These pores are cation selective, have a diameter of 1–2 nm and open in response to a transmembrane potential (Menestrina et al. 1995a). HlyA contributes to virulence through cytolysis of cells and a combination of secondary effects. These secondary effects have been proposed to have major contributions to bacterial virulence. The pore formation allows for passive influx of extracellular Ca2+ that, in nucleated cells, stimulates arachidonate metabolism leading to the production of lipid inflammatory mediators. Such mediators have deleterious effects on neighbouring cells resulting in organ dysfunction, release of granular constituents from granulocytes and release of pro-coagulatory substances from platelets.
The pore-forming function of HlyA is a conserved aspect of the haemolytic and leukotoxic mechanism of the RTX toxins (Benz et al. 1994; Clinkenbeard and Thiessen 1991; Maier et al. 1996; Menestrina et al. 1994; Schmidt et al. 1996). However, significant variation amongst the toxins exists such as the bifunctional toxin CyaA from Bor. pertussis (Glaser et al. 1988), which consists of a fusion of an adenylate cyclase domain and a pore-forming RTX moiety and recently identified multifunctional autoprocessing RTX toxins from V. cholerae (Lin et al. 1999). An unusual toxin is cytolysin (CylB) of Enterococcus faecalis, a causative agent of surgical wound infections (Callegan et al. 2002). CylB, which is exported by the ABC transporter CylT (Gilmore et al. 1990), is distantly related to bacteriocins and has been shown in numerous models to contribute to the severity of Ent. faecalis infections (Garsin et al. 2001; Huycke et al. 1991; Ike et al. 1984, 1987; Schlievert et al. 1997). The cytolysin operon is found in the Ent. faecalis pathogenicity island and is associated with a number of other virulence determinants, including aggregation substance and enterococcal surface protein.
Antibiotic and antimicrobial peptide efflux transporters
Numerous ABC transporters, functioning effectively as Type I secretion systems, have been identified in antibiotic, lantibiotic and bacteriocin-producing prokaryotes. These types of proteins do not mediate virulence towards mammalian species, but they should be mentioned as they are critical for inter- and intra-species competition against bacteria colonizing eukaryotic host niches. Thus, they can alter the behaviour and composition of the endogenous bacterial flora of the host. Notably, several ABC transporter genes have been observed to cluster with antibiotic biosynthetic genes. Examples of these ABC transporters include DrrAB, which secretes daunorubicin and doxorubicin, from Streptomyces peucetius (Guilfoile and Hutchinson 1991), the cyclic peptide antibiotic microcin J25 exporter McjD from E. coli (Solbiati et al. 1999), the OleC4-OleC5 oleandomycin transporter of Stp. antibioticus (Olano et al. 1995; Rodriguez et al. 1993), CmrAB of Stp. griseus which transports a chromoycin precursor (Menendez et al. 2007), the pyoluteorin efflux pump from Pseudomonas (Huang et al. 2006), kasugamycin resistance of Stp. kasugaensis (Ikeno et al. 2000), and the spiramycin transporter of Stp. ambofaciens (Schoner et al. 1992). In these organisms, it is highly likely that the primary physiological role of the ABC transporter is active efflux of the antibiotic, thereby providing self-resistance (Mendez and Salas 2001).
A number of ABC transporters involved in bacteriocin and lantibiotic export have been characterized in Gram-positive and Gram-negative bacteria, such as the Type I secretion system, CvaAB-TolC, which is dedicated to the export of the antibacterial toxin colicin V bacteriocin (Zhong et al. 1996). Colicin V kills sensitive bacterial cells by disrupting their membrane potential (Yang and Konisky 1984). There are numerous other ABC transporters associated with bacteriocin export such as the aureocin A70 transporter AurT from Sta. aureus (Netz et al. 2001), the PepT lanitibiotic exporter (Meyer et al. 1995) and epidermin secretion system (Peschel and Gotz 1996) of Sta. epidermidis, the subtilin transporters SpaB and SpaEFG of Bac. subtilis (Klein and Entian 1994), the nisin transporters NisT and NisFEG (Siegers and Entian 1995) and bacteriocin secretion system (Rince et al. 1997) of Lactococcus lactis, the macedocin exporter McdEFG from Str. macedonicus (Papadelli et al. 2007), the salivaricin exporter SboEFG from Str. salivarius (Hyink et al. 2007), and the gallidermin and epidermin transporter GdmT of Str. gallinarum (Hille et al. 2001). Some of these transporters may also provide some resistance to xenobiotics such as drugs. One notable example of this is the SmbG bacteriocin immunity protein from Str. mutans which is capable of providing resistance to antibiotics such as tetracycline and penicillin and is upregulated in response to exposure to these compounds (Matsumoto-Nakano and Kuramitsu 2006).
The non-proteinaceous β-exotoxin I is an unusual toxin that merits inclusion. Although it is produced by the Gram-positive insect pathogen Bac. thuringiensis, this Type I secreted toxin is highly toxic to mammalian cells (Beebee et al. 1972; Mackedonski et al. 1972). Bac. thuringiensis is often used as a biological control agent against insects. However, strains that produce β-exotoxin I, which mediates toxicity towards dipteran, coleopteran and lepidopteran species, cannot be used due to their potential toxicity towards humans and the persistence of toxin in the environment (Benz 1966). β-Exotoxin I is an adenine nucleotide analogue that is secreted by the ABC transporter BerAB (Espinasse et al. 2002). β-Exotoxin I is thought to act by inhibiting RNA polymerase function, which disrupts insect larval development (Sebesta and Horska 1970).
Proteases
The RTX family also includes the Type I-dependent secreted zinc metalloproteases (Hooper 1994; Miyoshi and Shinoda 2000) that are secreted by a variety of pathogens. These RTX proteases are ~50 kDa metzincin metalloendopeptidases that consist of an N-terminal proteolytic domain and a C-terminal calcium-binding RTX domain. The proteolytic domains share a conserved methionine, located on a turn near the catalytic site, and an elongated carboxy-terminal HEXXHXXGXXH motif. The three histidine residues in this motif are known to bind the catalytic zinc ion and the catalytically important glutamic acid residue (Stocker and Bode 1995; Stocker et al. 1995). RTX proteases involved in virulence have been identified in Pseudomonas (Duong et al. 1992; Guzzo et al. 1990; Liao and McCallus 1998); (Chabeaud et al. 2001; Woods et al. 2001) and Proteus (Walker et al. 1999; Wassif et al. 1995) species.
The RTX protease AprA from P. aeruginosa has been implicated in P. aeruginosa septicaemia (Fetzer et al. 1967) due to its proteolysis of fibrin and fibrinogen (Shibuya et al. 1991) and its anticoagulant activity in human plasma, which was attributed to its direct fibrinogenolytic function. AprA also readily cleaves soluble laminin (Heck et al. 1986), which suggests a direct role (together with elastase) in both tissue invasion and hemorrhagic tissue necrosis in P. aeruginosa infections. AprA has also been implicated in modulating the efficacy of the host immune response by proteolytically degrading human γ-interferon (Horvat and Parmely 1988) and components of serum complement (Hong and Ghebrehiwet 1992), the latter of which has been suggested to impair complement-mediated opsonization of P. aeruginosa. The RTX protease ZapA, from Proteus mirabilis, was observed to be involved in the proteolytic degradation of the immunoglobulins (Ig) IgG (Loomes et al. 1993), IgA1 and IgA2 (Wassif et al. 1995). The degradation of these Igs could confer protection against opsonization during urinary tract infections.
Surface layer proteins
The S-layer proteins form two-dimensional crystalline arrays that encompass the bacterial cell surface, playing roles in proteolysis, peptidoglycan catabolism, adhesion and immune evasion (Bahl et al. 1997; Beveridge et al. 1997). These surface arrays are self-assembling and are often composed of a single protein or glycoprotein species. The majority of bacterial S-layer proteins are exported via the Type II secretion pathway, but numerous Type I-dependent S-layer proteins, including some RTX family proteins, have also been identified. In C. rectus, a Gram-negative pathogen associated with human periodontal disease, three Type I secreted S-layer proteins, Crs, CsxA and CsxB, are known (Braun et al. 1999; LaGier and Threadgill 2008; Miyamoto et al. 1998; Wang et al. 1998). These proteins are proposed to be virulence factors and have been implicated in the evasion of host defences, such as phagocytosis and the bactericidal activity of serum (Okuda et al. 1997; Thompson 2002). C. fetus, a bacterial pathogen of ungulates and humans, also resists serum killing due to its S-layer which contains the Type I secreted SapA protein (Thompson et al. 1998)
Hemophore and siderophore secretion
Nutrient acquisition is a central facet of the host–pathogen interaction. Iron, which has important roles in many aspects of prokaryotic cellular metabolism and respiration, is highly constrained in the host environment, in part to restrict its availability to invading pathogens. Consequently, invasive pathogens utilize a range of mechanisms to scavenge and liberate iron from the host environment. Haemoglobin is one potential source of heme iron for bacterial pathogens. HasA is one of many secreted heme-binding proteins that scavenge free or protein-bound heme with high affinity and return it to bacterial cell surface receptors for use by the pathogen (Letoffe et al. 1994a). Serratia marcescens is an opportunistic pathogen responsible for a number of opportunistic and nosocomial infections, including those of the urinary and respiratory tracts, septicaemia and meningitis (Hejazi and Falkiner 1997). The hemoprotein from Ser. marcescens is secreted by an archetypal Type I secretion system comprised of the ABC transporter (HasD), a MFP (HasE) and an OMP of the TolC family (HasF) (Letoffe et al. 1994b). HasA is an atypical Type I secretion system allocrite because, unlike the majority of other translocated proteins, it requires a molecular chaperone, SecB, to prevent its folding in the cytoplasm prior to export (Debarbieux and Wandersman 2001; Delepelaire and Wandersman 1998). However, despite compelling biochemical data for the function of HasA, its contribution to bacterial pathogenicity remains ambiguous as a hasA deletion strain of Y. pestis (Rossi et al. 2001) showed no reduction in virulence. This is most likely due to the presence of additional iron scavenging pathways employed by pathogens.
Extracellular iron is also acquired by siderophores, such as pyoverdine (Pvd), which are known virulence factors of P. aeruginosa. This Fe-scavenging involves both ABC importer-mediated uptake (described in “Iron”) and, in P. aeruginosa, recycling of Pvd by an ABC exporter (Imperi et al. 2009). Upon binding of extracellular iron, Fe3+–Pvd is transported by the membrane-bound receptor FpvA into the periplasm, where the Fe3+ is reduced to Fe2+ and bound by an alternative binding protein for use within the cell (Greenwald et al. 2007; Schalk et al. 2002). Upon Fe2+ release, the iron-free siderophore, apo-Pvd, is recycled and secreted into the extracellular environment to scavenge additional iron. Recycling of Pvd is thought to occur due to the complex and energetically expensive production of the siderophore by non-ribosomal protein synthetic pathways (Andrews et al. 2003; Imperi et al. 2009). As a consequence, recycling of Pvd reduces cellular energy costs and avoids depletion of cellular resources. Pvd recycling occurs via the tripartite ABC efflux complex of PvdT–PvdR and OmpQ, which acts to efflux Pvd from the periplasm to the extracellular milieu where it associates with the membrane-bound FpvA receptor (Imperi et al. 2009). The contribution of this pathway to P. aeruginosa virulence was shown in growth competition assays where strains compromised for Pvd recycling were outcompeted by those competent for recycling (Griffin et al. 2004). Interestingly, both Pvd-producing and non-producing strains are isolated from biofilms in the lungs of cystic fibrosis patients (De Vos et al. 2001), which may suggest that competition for Pvd occurs within biofilm communities (Griffin et al. 2004).
Xenobiotic efflux
The active extrusion of xenobiotics is an essential protective mechanism for organisms from all domains of life to defend against dangerous compounds, whether endogenous metabolic by-products or pervasive environmental toxins that would otherwise damage organelles, DNA, proteins or cellular processes. Prokaryotic drug resistance facilitated by xenobiotic exporters is a by-product of this otherwise essential defence mechanism. Since the introduction of antibiotics in the 1930s, clinical drug resistance has reached the point where it has become so prevalent that overcoming bacterial resistance to drugs is now likened to an unwinnable war. Prokaryotes have an extraordinary array of different drug efflux systems, with many pathogens containing multiple copies of diverse drug efflux transporters. ABC exporters, renowned for their role in drug resistance in eukaryotic cancers, have also been implicated in contributing to prokaryotic drug resistance (Higgins 1992). The logic underlying this reasoning is based on the consistent ability of many ABC exporters to efflux a plethora of structurally unrelated compounds. However, individual ABC transporters have not yet been observed to be a major factor of prokaryotic multi-drug resistance (MDR) in mammalian pathogens. In this section, we will examine the function of ABC multi-drug transporters that are, by virtue of their function, often implicated in bacterial virulence.
LmrA
Multi-drug efflux by a bacterial ABC exporter was first demonstrated by the Gram-positive Lac. lactis transporter, LmrA. LmrA was identified on the basis of its high sequence homology with the human chemotherapeutic drug resistance ABC exporter ABCB1 (van Veen et al. 1996). LmrA transports allocrites that are partitioned into the inner leaflet of the cell membrane, thereby preventing compounds from accessing the cytoplasm and exhibiting cytotoxicity (Bolhuis et al. 1996). LmrA has a broad substrate range consistent with a polyspecific xenobiotic transporter (Poelarends et al. 2000). Heterologous expression of LmrA in E. coli confers resistance to a broad range of clinically relevant antibiotics, including those from the aminoglycoside, β-lactam, macrolide, quinolone and tetracycline families (Poelarends et al. 2002). Recent studies of LmrA have demonstrated H+–Na+–Cl− symport activity and this has led to the suggestion that symport function is most likely the physiological role of LmrA, allowing cells to survive in high osmolarity environments (Velamakanni et al. 2009).
BmrA
BmrA is an ABC efflux protein from the non-pathogenic Gram-positive bacterium Bac. subtilis that was identified on the basis of its significant homology (42% identity) to LmrA (Steinfels et al. 2004). BmrA is a homodimeric ABC efflux protein capable of transporting a range of allocrites including Hoechst 33342, mitoxantrone, 7-aminoatinomycin and doxorubicin (Chami et al. 2002; Dalmas et al. 2005; Ravaud et al. 2006; Steinfels et al. 2004). However, Bac. subtilis has not been observed to alter BmrA expression in response to ethidium bromide exposure during growth as has been observed for other multi-drug resistance transporters (Steinfels et al. 2004). This has led to the inference that BmrA may function as a constitutively expressed ABC efflux system responsible for the efflux of low levels of toxic compounds (Steinfels et al. 2004). Thus, the precise physiological role of BmrA in Bac. subtilis remains to be elucidated. Homologs of LmrA and BmrA have been identified through genomic screens of other organisms, such as HorA from Lactobacillus brevis (Sakamoto et al. 2001) and VcaM from the pathogen V. cholerae, but the majority of these ABC transporters lack detailed biochemical characterization and determination of their physiological functions.
LmrCD
LmrCD, also expressed by Lac. lactis, was the first characterized heterodimeric bacterial ABC multi-drug exporter (Lubelski et al. 2004). The role of LmrCD in multi-drug resistance has been implicated by the observation that expression of LmrCD was higher in four different MDR strains of Lac. lactis compared with the wild type (Lubelski et al. 2006a). LmrCD deletion mutants were also observed to be hypersensitive to a number of drug molecules including Hoechst 33342, rhodamine, cholate and daunomycin (Lubelski et al. 2006a). LmrC and LmrD are TMD–NBD fusion proteins but the NBDs are structurally distinct, with non-canonical residues present in the nucleotide-binding sites. Consequently, it has been suggested that the non-equivalence of binding sites in the NBDs establish distinct functional roles for these sites in the ATP hydrolysis process (Lubelski et al. 2006b). The physiological function of LmrCD has been proposed to be bile tolerance via the active efflux of cholate (Zaidi et al. 2008). Genomic screens have identified homologs of LmrCD in other bacterial species, such as EfrAB from Ent. faecalis (Lee et al. 2003), and although their actual contribution to in vivo drug resistance remains unclear, their role in bile tolerance may be important for pathogenic bacteria that colonize the intestine (Zaidi et al. 2008).
BilE
The bile exporter BilE is an atypical efflux pump of the human pathogen Listeria monocytogenes. Originally annotated as a glycine betaine/carnitine/choline ABC importer (Glaser et al. 2001), subsequent studies have shown that this ABC transporter is not involved in compatible solute-mediated environmental tolerance (Sleator et al. 2001) but has an important role in the active extrusion of toxic bile salts from the cytoplasm and is linked to virulence. Its similarity to the compatible solute family of transporters has been proposed to have arisen from the ability of BilE to sense environmental stimuli at the biochemical level in a manner similar to that of OpuA, through changes in membrane fluidity and stability. This could be a critical factor in Lis. monocytogenes ability to rapidly adapt to changes that perturb the bacterial membrane, the primary target of bile activity.
SmdAB
Ser. marcescens has a high level of intrinsic resistance to antibiotics similar to that of P. aeruginosa (Chen et al. 2003a). The genome of Ser. marcesens features a number of genes predicted to encode putative MDR pumps, including the ABC transporter SmdAB. Similar to LmrCD, SmdAB is thought to have functionally distinct NBDs (Matsuo et al. 2008). Studies of SmdAB indicate that it is capable of conferring resistance to a broad array of compounds including ciprofloxacin, norofloxacin, DAPI, tetracycline, Hoechst 33342 and TPPCI (Matsuo et al. 2008). However, despite its apparent in vitro capacity to mediate MDR, the role of SmdAB in virulence or in vivo drug resistance in this human pathogen has not yet been established.
Sav1866
Sav1866 from Sta. aureus is the only bacterial ABC transporter associated with multi-drug efflux for which high-resolution structural data is available. The structure of Sav1866 has been solved to 3.0 Å with ADP bound (Dawson and Locher 2006) and to 3.4 Å when complexed with the non-hydrolyzable ATP analogue AMP-PNP (Dawson and Locher 2007). By virtue of its canonical, homodimeric TMD–NBD arrangement, with each TMD comprised of six α-helices, Sav1866 is often viewed as the archetypal bacterial ABC exporter. However, despite the insights obtained from the high-resolution structure of Sav1866, knowledge of its actual role in MDR is more limited. To date, Sav1866 has been shown to be capable of exporting Hoechst 33342 and ethidium bromide (Velamakanni et al. 2008), but its role in in vivo antibiotic resistance remains to be elucidated. Consequently, although the structure of Sav1866 provides an ideal template for studies into bacterial multi-drug resistance ABC exporters, it may not be able to provide insights into the subtleties and differences found in all xenobiotic efflux proteins.
ABC importers associated with bacterial virulence
ABC importers facilitate the acquisition of essential compounds from the extracellular environment. This class of ABC transporters is found in prokaryotes, archaea and plants but is absent from other members of eukarya. ABC importers belong to the Class 3 subgroup of ABC transporters (Davidson et al. 2008) and have been further sub-divided by structural features into two classes, the Type I and Type II importers (Locher 2009). Type I ABC importers are associated with small molecule transport, such as ions and amino acids, and have five to eight TM helices in each TMD subunit. Type II importers are associated with large molecule transport, such as metal chelates and vitamins, and have ten TM helices in each TMD subunit (Locher et al. 2002). Although ABC importers show significant diversity in the number of TM helices, all ABC importers, with only a few notable exceptions, are synthesized as four distinct polypeptides, i.e. two NBD and two TMD polypeptides. In addition to the ABC transporter, a substrate binding protein (SBP) is required to form the ABC importer complex, also referred to as the ABC permease. However, a distinct class of non-canonical ABC importers that acquires compounds via a SBP-independent mechanism was recently identified (for a review, see Eitinger et al. (2011)). SBPs are high-affinity allocrite binding proteins with affinities that span from the nanomolar to the micromolar concentration range. Their role is to recruit and present the allocrite to the ABC importer. In general, SBPs are clustered within the same operon as the ABC transporter. However, microbial genome sequencing has identified numerous SBPs without an apparent cognate ABC transporter. These ‘orphan’ SBPs have recently been shown to interact with ABC transporters within pre-existing ABC permease complexes. This indicates that the transport capability of these complexes may be considerably larger than originally thought and is not solely governed by the SBP clustered within the operon (Chen et al. 2010). The nomenclature in this area warrants mention as it is an issue for potential confusion. The majority of genes have been named in sequential order such that the ‘A’ gene in some operons is the SBP, while in other transporters it refers to the NBD gene.
SBPs are responsible for the recruitment of a broad array of different compounds from the extracellular environment. Consequently, they have different structural and functional features to facilitate their roles (for a thorough review, see Berntsson et al. (2010)). In Gram-negative organisms, SBPs are located in the periplasmic compartment, while in Gram-positive organisms they are typically located on the external face of the cytoplasmic membrane tethered by a lipoprotein anchor or directly fused to the transporter (van der Heide and Poolman 2002). A similar organization exists in archaea, although some SBPs also have a type III signal sequence and have been proposed to form an extracellular oligomeric complex referred to as a ‘bindosome’ (Zolghadr et al. 2007). ABC permeases are often the primary uptake pathway for nutrients with limited extracellular concentrations. A central innate defensive mechanism employed in the host environment is the restriction of nutrient bioavailability to prevent colonization by pathogenic organisms. As a consequence, a number of ABC importers also have important roles in colonization and propagation of infection. In this section, we will review the ABC importers associated with bacterial virulence.
Metal ion acquisition
Nearly 30% of all proteins require interaction with a metal in order to facilitate biological activity (Andreini et al. 2008). The vast majority of metalloproteins use a first row transition metal, e.g. Mn, Fe, Cu or Zn. As a consequence, the host environment has evolved a number of strategies to restrict the bioavailability of these metal ions to concentration ranges of micromolar and below to limit the capacity for bacterial invasion and colonization. ABC importers associated with acquisition of the transition row metals Fe, Mn and Zn are highly represented in studies of bacterial pathogenicity.
Iron
Iron was integrated into biological processes early in evolution due to its environmental abundance and range of different oxidation states. In a biological context, iron has key roles in a range of pathways by virtue of its flexible redox chemistry including respiration, photosynthesis and nitrogen fixation. Iron acquisition is also of major importance for bacterial pathogens and, consequently, the animal or human host employs a range of strategies to limit its bioavailability (in the order of ~10−18 M) (Braun 2001), such as sequestering it in lactoferrin or transferrin to restrict its availability to non-host processes. There are three classes of iron-specific uptake systems: the ferric iron transporters (TCID 3.A.1.10), the iron chelate transporters (3.A.1.14) and the ferric siderophore uptake transporters (3.A.1.21). In addition, some members of the Mn/Zn/Fe chelate transporter family (3.A.1.15) have been implicated in iron acquisition.
The ferric transporters facilitate the import of ions in a Fe3+ chelate/siderophore-independent manner into the cytosol. The Fe3+-ABC permeases, FbpABC in N. gonorrhoeae and meningitidis (Adhikari et al. 1996; Chen et al. 1993) and HitABC in H. influenzae (Adhikari et al. 1995; Sanders et al. 1994), employ the Fe3+ binding protein, FbpA or HitA, respectively, to recruit Fe3+ ions to the ABC transporter FbpBC or HitBC. In Neisseria spp., the affinity of FbpA is such that it is capable of stripping the Fe3+ directly from transferrin for use by the pathogen (Anderson et al. 2004; Shouldice et al. 2004; Taboy et al. 2001). As Fe3+ ions would not occur, under normal physiological conditions, in a ‘free’ or unbound state, it is likely that in pathogenic bacteria, ABC permeases of this class would be involved in directly acquiring Fe3+ from host proteins and carrier molecules.
The iron chelate transporters and ferric–siderophore transporters are responsible for the acquisition of iron from the host via a bacterial chelating compound. A variety of different chelating siderophores are known and the ABC permease import-dependent siderophores include ferric-hydroxamates, -bactins and -dicitrates. In Gram-positive bacteria, the ABC permease import process involves an extracellular SBP that binds the siderophore that then delivers it via its ABC transporter into the cytosol. In Gram-negative bacteria, as the siderophores are too large for passage through the OM porins, they interact with an outer membrane transporter for delivery into the periplasmic compartment via a TonB system (Faraldo-Gomez and Sansom 2003; Paulsen et al. 1997; Postle 1990; Wang and Newton 1971), whereupon they are then able to interact with a siderophore-specific SBP and its cognate ABC transporter (Sutcliffe and Russell 1995). In both Gram-positive and -negative bacteria, the iron-bound siderophore interacts with cytosolic proteins, causing the release of iron. The archetypal Gram-negative siderophore ABC permease is the FepBCDG system of E. coli which transports enterobactin. In this pathway, the Fe3+-loaded siderophore is bound on the outer membrane by FepA (Braun et al. 1998; Buchanan et al. 1999). The ferric–siderophore complex is then translocated into the periplasm in a process involving TonB, ExbB and ExbD (Moeck and Coulton 1998), where it is bound by the SBP FepB (Braun et al. 1998). FepB delivers the ferric–siderophore complexes to the ABC transporter FepCDG (Chenault and Earhart 1991; Shea and McIntosh 1991). Once localized to the cytoplasm, Fe3+ is removed from the siderophore by a cytoplasmic enzyme, Fes.
The iron chelate transporters (TCID 3.A.1.21), as typified by the archetypal E. coli FepBCDG or FecBCDE iron chelate transporters, have been observed in the genomic screens of almost all prokaryotes. Notably, in studies of pathogenic bacteria, these transporters often contribute to the virulence capacity of these organisms. Notable examples include the PiaABCD ABC permease of Str. pneumoniae (Brown et al. 2001), the ABC permease FtsABC of Str. pyogenes (Hanks et al. 2005), the HtsABC, FhuCBG-D1-D2, SirABC and SstABCD ABC permeases of Sta. aureus (Beasley et al. 2009; Dale et al. 2004; Morrissey et al. 2000; Speziali et al. 2006) and the ViuPDCG ABC permease of V. cholera (Butterton et al. 1992; Henderson and Payne 1993, 1994; Occhino et al. 1998; Wyckoff et al. 1999).
A subset of these transporters is involved in the scavenging of iron from haemoglobin released by the Type I secreted toxin HlyA from erythrocytes. The HlyA-induced lysis of erythrocytes allows secreted hemophores, such as HasA, to bind heme groups released from haemoglobin degradation (Cescau et al. 2007). Hemophore transporters then either strip the iron from the hemophores directly or transport them into the cells, where the porphyrin ring is opened by oxygenase to release iron (Tong and Guo 2009). These ABC-dependent heme transporters have been identified in a number of virulence studies of pathogenic organisms such as Str. pyogenes (HtsABCD or SiaABC) (Bates et al. 2003; Lei et al. 2003; Nygaard et al. 2006; Sun et al. 2010), V. cholerae (HutABCD) (Henderson and Payne 1993, 1994; Occhino et al. 1998), P. aeruginosa (PhuSTUVW) (Ochsner et al. 2000; Tong and Guo 2009), Shigella dysenteriae (ShuSTUV) (Burkhard and Wilks 2008) and Sta. aureus (HtsABC) (Grigg et al. 2007; Tong and Guo 2009).
Y. pestis has three known siderophore transport systems, two of which have been shown to be associated with virulence. The iron chelate transport pathway YfeABCD, which transports the siderophore yersiniabactin, employs the SBP YfeA to acquire yersiniabactin in the periplasm, which is then transported into the cytoplasm via YfeBCD (Perry et al. 2007). Strains lacking a functional YfeBCD ABC permease were avirulent in mouse models (Bearden and Perry 1999). The second yersiniabactin transporter identified is the YbtPQ ABC permease. This transporter belongs to the ferric–siderophore uptake group (TCID 3.A.1.21) and is structurally unique among ABC importers as it is comprised of two fusion polypeptides featuring an amino-terminal TMD and a carboxy-terminal NBD. In Y. pestis, loss of this ABC permease also resulted in an attenuation of virulence in a mouse infection model (Fetherston et al. 1999). Intriguingly, although both transporters translocate yersiniabactin into the cytoplasm, loss of either ABC permease was sufficient to attenuate in vivo virulence in their respective mouse models. This indicates that, during infection, iron acquisition by Y. pestis is essential for invasion and/or colonization of the host and that both pathways are necessary for this process. As iron accumulation was reduced, but not abrogated in both studies, this could suggest that yersiniabactin uptake is a rate-limited step. A homolog of the YbtPQ system has also been identified in M. tuberculosis, the IrtAB ABC permease. Loss of this pathway resulted in decreased survival in a mouse lung in vivo infection model (Rodriguez and Smith 2006; Ryndak et al. 2010).
In addition to the major classes of iron transporters, members of the Mn/Zn/Fe chelate transporters (3.A.1.15) are also involved in iron acquisition. However, phenotypic studies of this class of transporters indicate that its members are predominantly involved in manganese and/or zinc acquisition and, consequently, the actual physiological contribution to iron acquisition is less certain. Despite this, there is evidence that these transporters are regulated in response to extracellular iron concentrations, for example, in Salmonella Typhimurium, where the SitABCD ABC permease transporter was up-regulated under iron-restricted conditions (Cockayne et al. 1998) and deletion of the ABC permease genes attenuated virulence in a mouse model (Janakiraman and Slauch 2000; Wandersman and Delepelaire 2004). However, these studies did not unequivocally demonstrate that SitABCD was required for iron acquisition in vivo. Similarly, in Sta. epidermidis, the SitABCD permease which is capable of both iron and manganese uptake is expressed during the implantation phase of infection (Massonet et al. 2006), but the metal transported in vivo was not established.
The studies of iron transport systems in pathogenic bacteria, in general, indicate that although multiple iron transport pathways exist, loss of a single one is often sufficient to reduce or attenuate virulence in vivo. This has great significance for understanding how pathogens respond to the limitation of essential nutrients during invasion and colonization of the host.
Manganese
Although Mn is essential for almost all forms of life, its biological importance was overlooked for many years. Mn has known roles in a wide range of enzymes involved in phosphorylation, hydrolysis, carbon metabolism, decarboxylation and oxidative stress response (Papp-Wallace and Maguire 2006). More recently, its importance in bacterial pathogenesis has become increasingly apparent, where it is implicated in having a number of roles complementary to those of iron. Mn ABC permeases belong to the broad family of Mn/Zn/Fe chelate transporters (3.A.1.15). ABC permeases involved in Mn transport were first identified in Gram-positive bacteria as putative adhesion proteins belonging to the lipoprotein receptor-associated antigen I family (Jenkinson 1994). Subsequent studies found that they also had the capacity to function as specific Mn transporters (Kitten et al. 2000; Kolenbrander et al. 1998). Since then, ABC permeases of the Mn/Zn/Fe chelate transporter family have been identified in almost all pathogenic bacteria, but in many cases the physiological function of these transporters remains nebulous and inferred largely based on sequence comparisons of the SBPs. An additional complication in establishing the significance of this group of permeases is that many early studies often misidentified these transporters due to their ability to bind or transport other metal ions, albeit at lower affinities than their cognate ligand (Janakiraman and Slauch 2000).
The best-characterized Mn transporter is the PsaBCA permease from the major human pathogen Str. pneumoniae (Berry and Paton 1996; Dintilhac et al. 1997; Lawrence et al. 1998; McAllister et al. 2004; Tseng et al. 2002). Its role in Mn transport has been definitively established and loss of any component of this Mn uptake system has been shown to compromise growth on Mn-restricted media and results in complete attenuation of virulence in vivo (McAllister et al. 2004; McDevitt et al. 2011). Similar phenotypes have been observed in Str. pyogenes, where deletion of the high-affinity Mn ABC permease MtsABC resulted in a 30-fold reduction in virulence in a mouse infection model, and in Str. mutans, where deletion of the Mn ABC permease SloABC reduced endocarditis virulence in a rat infection model (Paik et al. 2003). Similar findings were observed in Str. suis where loss of the Mn SBP TroA in a murine Str. suis infection model resulted in an avirulent phenotype (Schreur et al. 2011) and in N. gonorrhoeae (MntABC) where permease was required for biofilm formation and intracellular survival (Lim et al. 2008).
In addition to the Mn ABC permease, some prokaryotes also utilize natural resistance-associated macrophage protein (NRAMP) systems as secondary Mn transporters. However, these proton-coupled transporters have not, in general, been found to serve as primary Mn uptake pathways. As a consequence, the requirement of the Mn ABC permease for virulence in organisms that also possess a NRAMP system is not unequivocal. Nevertheless, the overall trend in the literature suggests that in the majority of cases the ABC permease is the primary virulence determinant. In Sta. aureus, which possesses both a high-affinity Mn ABC permease (MntABC) and a NRAMP system (MntH), phenotypic studies found that the ABC permease was the high-affinity Mn transporter (Horsburgh et al. 2002). However, in a mouse abscess model, both systems had to be disrupted before a reduction in virulence was observed (Ando et al. 2003; Horsburgh et al. 2002). In contrast, in the Gram-negative pathogen Salmonella Typhimurium, mutation of the Mn permease SitABCD, alone or in conjunction with the NRAMP system, reduced virulence in a Nramp1 G169 murine typhoid model of infection (Kehres et al. 2002; Zaharik et al. 2004). Similarly, in avian pathogenic E. coli O78 strain chi7122, the SitABCD transporter was found to be more important for virulence in a chicken infection model than the NRAMP system (Sabri et al. 2008).
The recurring theme from these studies is that the Mn ABC permease has an important role in protection from oxidative stress and that in vivo loss of this function is associated with a compromised ability to mediate virulence. This strongly indicates that, during host colonization, many pathogens have a critical dependence on Mn acquisition to manage oxidative stress independent of iron-based protective mechanisms. Furthermore, this is significant in both aerobes and facultative aerobes and suggests that it may not simply be due to a compromised ability to effectively manage oxidative stress arising from growth under aerobic conditions but may also have a role in protection from exogenous redox stress mediated by innate immune defences.
Zinc
Zn is also an essential metal ion and is highly abundant in biology due to its single oxidation state in solution (Andreini et al. 2006b). In humans, it is the second most abundant transition row element and recent studies have suggested that zinc-binding proteins could constitute about 10% of the entire human proteome (Andreini et al. 2006a). Zn has critical structural and/or catalytic roles in all the major classes of enzymes and is also important for transcription and replication factors (Andreini et al. 2008; Coleman 1998). As a consequence, Zn acquisition in vivo has been found to be a virulence determinant in a number of pathogenic bacteria.
The Zn ABC permease, ZnuABC, has been observed to be involved in the virulence of E. coli O157:H7 (Gabbianelli et al. 2011), E. coli CFT073 (Gunasekera et al. 2009; Sabri et al. 2009) and Salmonella Typhimurium (Ammendola et al. 2007; Campoy et al. 2002). In addition to the Zn ABC permease, an additional Zn recruitment factor ZinT, or YodA, has been found in some Gram-negative pathogens, such as Salmonella Typhimurium, where it is thought to function as a Zn chaperone aiding in the recruitment of Zn to ZnuA in Zn-restricted environments (Petrarca et al. 2010). The Zn ABC permease in Str. pneumoniae, although not independently shown to mediate virulence (Claverys 2001), has an unusual organization. The permease is encoded by the adcABC operon that includes the high-affinity Zn SBP AdcA. However, a second Zn SBP AdcAII was recently identified clustered with a group of cell surface-localized histidine triad proteins expressed in response to Zn restriction (Loisel et al. 2008). The physiological significance of this apparent redundancy in Zn recruitment is currently unclear. Intriguingly, Str. pyogenes encodes an orphan Zn-binding protein, Lsp, that is homologous to AdcAII both in sequence and genomic context, i.e. clustered with the histidine triad proteins. Mutation of the Lsp metal-binding site leads to a decrease in virulence in mouse soft tissue, implicating an important role for Lsp in Zn homeostasis. Intriguingly, Str. pyogenes lacks a homolog of the primary Zn SBP AdcA, which is clustered with the cognate ABC transporter AdcBC in Str. pneumoniae. As Str. pyogenes retains a homolog of the ABC transporter, it is likely that Lsp mediates Zn transport through AdcBC, but this has not yet been demonstrated (Weston et al. 2009). The specific requirement of Zn to mediate virulence, if any, remains unclear. However, in addition to its general requirement for enzyme structure and function, it has been suggested that it is required for activation of copper/zinc superoxide dismutase in invasive pathogens to help defend against host-induced oxidative and nitrosative stress (Claverys 2001).
Amino acid transporters
Prokaryotes acquire amino acids from the host environment to use as carbon and/or nitrogen sources or because they are required due to auxotrophic growth conditions. Although amino acid transporters have been observed to be up-regulated in numerous bacterial pathogens in response to colonization of the host, there are only a few specific examples of amino acid ABC permeases being required for survival in the host environment. The glutamine (Gln) ABC permease GlnPQ has been observed to be required for Str. pneumoniae (Hartel et al. 2011) and Group B Streptococcus (GBS). The Str. pneumoniae genome encodes six Gln ABC permeases, two of which lack the putative Gln SBP GlnH, which is suggestive of significant functional redundancy. However, in vivo studies of two of these transporters found that deletion of a single Gln ABC permease significantly affected virulence, resulting in moderate to near-complete attenuation (Hartel et al. 2011). In GBS, loss of the GlnPQ ABC permease resulted in decreased in vitro and in vivo virulence due to impaired fibronectin adherence, although the underlying relationship between cellular Gln levels and fibronectin adhesins remains unclear. C. jejuni also contains a Gln ABC permease, PaqPQ, but strangely, loss of this permease was associated with an enhancement of resistance to aerobic and organic peroxide stress and an improved survival in intracellular macrophage and epithelial cell models. Other amino acid transporters have been observed to have roles in the virulence of specific pathogens, such as the glutamate (Glu) transporter of N. meningitidis GlnT (Colicchio et al. 2009), and no doubt more will be uncovered through targeted in vivo studies.
Peptide transporters
In addition to amino acids, another source of carbon and/or nitrogen utilized by prokaryotes are peptides that are acquired from the environment by di- or tripeptide (Dpp) ABC permeases and/or oligopeptide (Opp) ABC permeases belonging to the peptide/opine uptake family (TCID 3.A.1.5). In addition to their role in cellular metabolism, these peptide ABC permeases are also implicated in signalling processes and in virulence (reviewed in Detmers et al. (2001) and Doeven et al. (2005)). In Group A Streptococcus (GAS) strain CS101, the Dpp ABC permease is associated with uptake of amino acids, heme production, chemotaxis, sporulation and expression of the SpeB cysteine protease, a major virulence factor. In the human pathogen Str. agalactiae, the Opp ABC permease was observed to be involved in regulating virulence, observed as a decrease in adherence to epithelial cells, via reduced expression of fibrinogen-binding protein, FbsA. These observations show that peptide acquisition has an important role in pathogen invasion, although the mechanism, possibly through environmental sensing, remains to be determined.
Vitamins
In the host, vitamin B12 has an important role in natural killer cell activation and lymphocyte production. Bacteria have been proposed to scavenge vitamin B12 from the host environment and, thereby, reduce the hostility of the host environment for themselves (Tamura et al. 1999). The TonB-dependent vitamin B12 importer BtuCED (TCID 3.A.1.13) has been identified in E. coli (de Veaux et al. 1986) and imports vitamin B12 in corrinoids (Borths et al. 2002; Chimento et al. 2003; Locher et al. 2002) to the periplasmic binding protein BtuF (Hvorup et al. 2007). However, the requirement of a vitamin B12 ABC permease for full virulence has not yet been established in an in vivo infection model.
Osmoprotectants
Often during infection, there is increased osmotic pressure at the site of infection. Consequently, to avoid membrane damage from osmotic stress, bacteria acquire osmoprotectants from the environment. These include proline, choline, ectoine, glycine betaine and proline betaine, which are acquired by ABC permeases (TCID 3.A.1.12). In Lac. lactis, OpuABC imports glycine betaine and proline betaine from the extracellular environment (Horn et al. 2005, 2006; van Der Heide and Poolman 2000). These osmolyte transporters are present in a diverse range of human pathogens, such as M. tuberculosis, which expresses the ProXVWZ betaine transporter (Price et al. 2008), and P. aeruginosa, which expresses the ABC permease CbcXWV that has been shown to import choline, betaine and carnitine (Chen et al. 2010). In Sta. aureus, the choline ABC permease OpuABC was found to be important for resistance to salt stress via choline uptake. Loss of the ABC permease rendered Sta. aureus less pathogenic and defective in biofilm formation in vitro and in vivo (Kiran 2009). In Lis. monocytogenes, growth at high salt concentrations is attributed to the accumulation of organic solutes such as glycine betaine and carnitine. In the Lis. monocytogenes LO28 strain, a deletion of three of its osmolyte ABC permease systems (BetL, Gbu and OpuC) resulted in a significant reduction in its ability to cause systemic infection following peroral coinoculation with the wild-type parent in a murine model (Wemekamp-Kamphuis et al. 2002). However, deletion of OpuC alone resulted in similar effects (Sleator et al. 2001), leading to the inference that the OpuABC permease may play an important role in listerial pathogenesis (Wemekamp-Kamphuis et al. 2002).
Antimicrobial peptides
Animals and humans produce small cationic peptides of varying lengths and sequences with antimicrobial properties, which assist in a strong non-specific innate immune response. Such peptides include protamine, melitlin and polymyxin B. It is thought that these peptides destabilize the outer cytoplasmic membrane of pathogens. Many pathogens have evolved methods to withstand the effects of these cationic peptides (Otto 2009) via ABC permeases (TCID 3.A.1.5.1), which internalize these peptides for detoxification. Salmonella Typhimurium has two known systems: the first, SapABCDF, has been associated with protamine detoxification, involving SapA binding the peptide and delivering it to the ABC permease SapBCDF to be internalized (Groisman 1994; Groisman et al. 1992; Parra-Lopez et al. 1993). Once acquired, the current models hypothesize that the bacteria decomposes the peptide, rendering it ineffective. The second known system is the YjeABEF ABC permease that confers resistance to protamine, melittin, polymyxin B and human defensin-1 and -2. Mutations in this permease decreased proliferation in activated macrophages and attenuated survival in a mouse typhoid fever model (Eswarappa et al. 2008). The presence of two permeases in Salmonella Typhimurium indicates that there is functional overlap of these antimicrobial peptide transporters, but the significance of this has not been established outside of their respective infection models. Examples of similar antimicrobial peptide ABC permeases have been found in other pathogens. For example in H. influenzae, the sap operon was upregulated when exposed to cationic peptides and was found to be required for resistance (Mason et al. 2006). In E. coli, the YejABEF importer has also been identified, but a direct role in the virulence of pathogenic strains has not been established (Novikova et al. 2007; Stumpe and Bakker 1997).
Other transporters
In addition to the general classes of transporters above, there is also an increasing number of ABC permeases that have been found to have specialized functions associated with the virulence of pathogenic bacteria. These include M. tuberculosis and the ABC permease LpqY/SugABC responsible for the import of the disaccharide trehalose. Trehalose, which is not present in mammals, arises as a byproduct of mycolic acid biosynthesis, a key constituent of the mycobacterial cell envelope. The trehalose ABC permease is proposed to mediate the retrograde transport of released trehalose, thereby functioning as a recycling system. Perturbation in this recycling process strongly impaired M. tuberculosis virulence, implying a central role for the trehalose ABC permease in pathogenicity (Kalscheuer et al. 2010).
Conclusions
Prokaryotic ABC transporters show a remarkable diversity of functions, some of which are essential for the virulence of pathogenic bacteria. However, the physiological function of many ABC transport systems remains to be elucidated. Indeed many of the biochemically characterized systems, such as the xenobiotic transporters, may have distinct primary physiological functions that supersede their observed roles in drug efflux. Intriguingly, although ABC transporters have a clear contribution to virulence, in contrast to the role of ABC transporters in eukaryotic diseases, these roles are rarely associated with multi-drug efflux. In pathogenic bacteria, these roles are often evasion of or resistance to host defences, coupled with functions that aid colonization of the host environment, such as nutrient scavenging. As a consequence, many of the cell surface or secreted factors may prove to be useful targets for antimicrobial therapeutics or vaccine development. However, their applications in these roles would rely on a clear understanding of their physiological function and role in virulence, and so clearly more research into this ‘frontier territory’ of proteins is needed.
Abbreviations
- ABC:
-
ATP-binding cassette
- Bac. :
-
Bacillus
- Bor. :
-
Bordetella
- C. :
-
Campylobacter
- CM:
-
Cytoplasmic membrane
- CPS:
-
Capsular polysaccharides
- Da:
-
Daltons
- DPP:
-
di- or tripeptide ABC permeases
- E. :
-
Escherichia
- Ent. :
-
Enterococcus
- EPS:
-
Exopolysaccharide
- GAS:
-
Group A Streptococcus
- GBS:
-
Group B Streptococcus
- Gln:
-
Glutamine
- Glu:
-
Glutamate
- H. :
-
Haemophilus
- Hly:
-
α-Hemolysin
- Ig:
-
Immunoglobulin
- Lac. :
-
Lactococcus
- Lis. :
-
Listeria
- LPS:
-
Lipopolysaccharide
- M. :
-
Mycobacterium
- MFP:
-
Membrane fusion protein
- MDR:
-
Multi-drug resistance
- N. :
-
Neisseria
- NBD:
-
Nucleotide-binding domains
- NRAMP:
-
Natural resistance-associated macrophage protein
- O:
-
Oligosaccharide
- O-units:
-
Oligosaccharide units
- OMP:
-
Outer membrane porin
- Opp:
-
Oligopeptide ABC permeases
- P. :
-
Pseudomonas
- PG:
-
Peptidoglycan
- Pvd:
-
Pyoverdin
- RTX:
-
Repeats-in-toxin
- SBP:
-
Substrate-binding protein
- S:
-
Surface
- Ser.:
-
Serratia
- Sta. :
-
Staphylococcus
- Stp. :
-
Streptomyces
- Str. :
-
Streptococcus
- TC:
-
Transporter classification
- TCID:
-
Transporter classification identification
- TM:
-
Transmembrane
- TMD:
-
Transmembrane domain
- V. :
-
Vibrio
- Y. :
-
Yersinia
References
Achtman M, Pluschke G (1986) Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol 40:185–210
Adhikari P, Kirby SD, Nowalk AJ, Veraldi KL, Schryvers AB, Mietzner TA (1995) Biochemical characterization of a Haemophilus influenzae periplasmic iron transport operon. J Biol Chem 270:25142–25149
Adhikari P, Berish SA, Nowalk AJ, Veraldi KL, Morse SA, Mietzner TA (1996) The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J Bacteriol 178:2145–2149
Alaimo C et al (2006) Two distinct but interchangeable mechanisms for flipping of lipid-linked oligosaccharides. EMBO J 25:967–976
Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A (2007) High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun 75:5867–5876
Anderson DS, Adhikari P, Nowalk AJ, Chen CY, Mietzner TA (2004) The hFbpABC transporter from Haemophilus influenzae functions as a binding-protein-dependent ABC transporter with high specificity and affinity for ferric iron. J Bacteriol 186:6220–6229
Ando M, Manabe YC, Converse PJ, Miyazaki E, Harrison R, Murphy JR, Bishai WR (2003) Characterization of the role of the divalent metal ion-dependent transcriptional repressor MntR in the virulence of Staphylococcus aureus. Infect Immun 71:2584–2590
Andreini C, Banci L, Bertini I, Rosato A (2006a) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5:196–201
Andreini C, Banci L, Bertini I, Rosato A (2006b) Zinc through the three domains of life. J Proteome Res 5:3173–3178
Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218
Andrews SC, Robinson AK, Rodriguez-Quinones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237
Angelos JA, Hess JF, George LW (2003) An RTX operon in hemolytic Moraxella bovis is absent from nonhemolytic strains. Vet Microbiol 92:363–377
Arrecubieta C, Hammarton TC, Barrett B, Chareonsudjai S, Hodson N, Rainey D, Roberts IS (2001) The transport of group 2 capsular polysaccharides across the periplasmic space in Escherichia coli. Roles for the KpsE and KpsD proteins. J Biol Chem 276:4245–4250
Bahl H et al (1997) Molecular biology of S-layers. FEMS Microbiol Rev 20:47–98
Bakkes PJ, Jenewein S, Smits SH, Holland IB, Schmitt L (2010) The rate of folding dictates substrate secretion by the Escherichia coli hemolysin type 1 secretion system. J Biol Chem 285:40573–40580
Bates CS, Montanez GE, Woods CR, Vincent RM, Eichenbaum Z (2003) Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect Immun 71:1042–1055
Baumann U, Wu S, Flaherty KM, McKay DB (1993) Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J 12:3357–3364
Bearden SW, Perry RD (1999) The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol 32:403–414
Beasley FC et al (2009) Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol Microbiol 72:947–963
Becker A, Kuster H, Niehaus K, Puhler A (1995) Extension of the Rhizobium meliloti succinoglycan biosynthesis gene cluster: identification of the exsA gene encoding an ABC transporter protein, and the exsB gene which probably codes for a regulator of succinoglycan biosynthesis. Mol Gen Genet 249:487–497
Beebee T, Korner A, Bond RP (1972) Differential inhibition of mammalian ribonucleic acid polymerases by an exotoxin from Bacillus thuringiensis. The direct observation of nucleoplasmic ribonucleic acid polymerase activity in intact nuclei. Biochem J 127:619–634
Bengoechea JA, Najdenski H, Skurnik M (2004) Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol Microbiol 52:451–469
Benz G (1966) On the chemical nature of the heat-stable exotoxin of Bacillus thuringiensis. Experientia 22:81–82
Benz R, Hardie KR, Hughes C (1994) Pore formation in artificial membranes by the secreted hemolysins of Proteus vulgaris and Morganella morganii. Eur J Biochem 220:339–347
Berntsson RP, Smits SH, Schmitt L, Slotboom DJ, Poolman B (2010) A structural classification of substrate-binding proteins. FEBS Lett 584:2606–2617
Berry AM, Paton JC (1996) Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun 64:5255–5262
Beutler B, Poltorak A (2000) The search for Lps: 1993–1998. J Endotoxin Res 6:269–293
Beveridge TJ et al (1997) Functions of S-layers. FEMS Microbiol Rev 20:99–149
Biemans-Oldehinkel E, Doeven MK, Poolman B (2006) ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett 580:1023–1035
Bolhuis H, van Veen HW, Molenaar D, Poolman B, Driessen AJ, Konings WN (1996) Multidrug resistance in Lactococcus lactis: evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J 15:4239–4245
Borths EL, Locher KP, Lee AT, Rees DC (2002) The structure of Escherichia coli BtuF and binding to its cognate ATP binding cassette transporter. Proc Natl Acad Sci U S A 99:16642–16647
Braun V (2001) Iron uptake mechanisms and their regulation in pathogenic bacteria. Int J Med Microbiol 291:67–79
Braun V, Hantke K, Koster W (1998) Bacterial iron transport: mechanisms, genetics, and regulation. Met Ions Biol Syst 35:67–145
Braun M, Kuhnert P, Nicolet J, Burnens AP, Frey J (1999) Cloning and characterization of two bistructural S-layer-RTX proteins from Campylobacter rectus. J Bacteriol 181:2501–2506
Bronner D et al (1993) Synthesis of the K5 (group II) capsular polysaccharide in transport-deficient recombinant Escherichia coli. FEMS Microbiol Lett 113:279–284
Brown JS, Gilliland SM, Holden DW (2001) A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol Microbiol 40:572–585
Buchanan SK et al (1999) Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol 6:56–63
Burkhard KA, Wilks A (2008) Functional characterization of the Shigella dysenteriae heme ABC transporter. Biochemistry 47:7977–7979
Butterton JR, Stoebner JA, Payne SM, Calderwood SB (1992) Cloning, sequencing, and transcriptional regulation of viuA, the gene encoding the ferric vibriobactin receptor of Vibrio cholerae. J Bacteriol 174:3729–3738
Callegan MC, Engelbert M, Parke DW 2nd, Jett BD, Gilmore MS (2002) Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium–host interactions. Clin Microbiol Rev 15:111–124
Campoy S, Jara M, Busquets N, Perez De Rozas AM, Badiola I, Barbe J (2002) Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar Typhimurium virulence. Infect Immun 70:4721–4725
Cescau S, Cwerman H, Letoffe S, Delepelaire P, Wandersman C, Biville F (2007) Heme acquisition by hemophores. Biometals 20:603–613
Chabeaud P, de Groot A, Bitter W, Tommassen J, Heulin T, Achouak W (2001) Phase-variable expression of an operon encoding extracellular alkaline protease, a serine protease homolog, and lipase in Pseudomonas brassicacearum. J Bacteriol 183:2117–2120
Chami M, Steinfels E, Orelle C, Jault JM, Di Pietro A, Rigaud JL, Marco S (2002) Three-dimensional structure by cryo-electron microscopy of YvcC, an homodimeric ATP-binding cassette transporter from Bacillus subtilis. J Mol Biol 315:1075–1085
Chang YF, Young R, Struck DK (1989) Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA 8:635–647
Chang YF, Ma DP, Young R, Struck DK (1993) Cloning, sequencing and expression of a Pasteurella haemolytica A1 gene encoding a PurK-like protein. DNA Seq 3:357–367
Chen CY, Berish SA, Morse SA, Mietzner TA (1993) The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol Microbiol 10:311–318
Chen J, Lee EW, Kuroda T, Mizushima T, Tsuchiya T (2003a) Multidrug resistance in Serratia marcescens and cloning of genes responsible for the resistance. Biol Pharm Bull 26:391–393
Chen J, Lu G, Lin J, Davidson AL, Quiocho FA (2003b) A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell 12:651–661
Chen C, Malek AA, Wargo MJ, Hogan DA, Beattie GA (2010) The ATP-binding cassette transporter Cbc (choline/betaine/carnitine) recruits multiple substrate-binding proteins with strong specificity for distinct quaternary ammonium compounds. Mol Microbiol 75:29–45
Chenault SS, Earhart CF (1991) Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol Microbiol 5:1405–1413
Chimento DP, Mohanty AK, Kadner RJ, Wiener MC (2003) Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat Struct Biol 10:394–401
Clarke BR, Cuthbertson L, Whitfield C (2004) Nonreducing terminal modifications determine the chain length of polymannose O antigens of Escherichia coli and couple chain termination to polymer export via an ATP-binding cassette transporter. J Biol Chem 279:35709–35718
Claverys JP (2001) A new family of high-affinity ABC manganese and zinc permeases. Res Microbiol 152:231–243
Clinkenbeard KD, Thiessen AE (1991) Mechanism of action of Moraxella bovis hemolysin. Infect Immun 59:1148–1152
Cockayne A, Hill PJ, Powell NB, Bishop K, Sims C, Williams P (1998) Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun 66:3767–3774
Coleman JE (1998) Zinc enzymes. Curr Opin Chem Biol 2:222–234
Colicchio R et al (2009) The meningococcal ABC-Type l-glutamate transporter GltT is necessary for the development of experimental meningitis in mice. Infect Immun 77:3578–3587
Cuthbertson L, Powers J, Whitfield C (2005) The C-terminal domain of the nucleotide-binding domain protein Wzt determines substrate specificity in the ATP-binding cassette transporter for the lipopolysaccharide O-antigens in Escherichia coli serotypes O8 and O9a. J Biol Chem 280:30310–30319
Cuthbertson L, Mainprize IL, Naismith JH, Whitfield C (2009) Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol Mol Biol Rev 73:155–177
Cuthbertson L, Kos V, Whitfield C (2010) ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev 74:341–362
Dale SE, Sebulsky MT, Heinrichs DE (2004) Involvement of SirABC in iron-siderophore import in Staphylococcus aureus. J Bacteriol 186:8356–8362
Dalmas O, Orelle C, Foucher AE, Geourjon C, Crouzy S, Di Pietro A, Jault JM (2005) The Q-loop disengages from the first intracellular loop during the catalytic cycle of the multidrug ABC transporter BmrA. J Biol Chem 280:36857–36864
Dassa E, Bouige P (2001) The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms. Res Microbiol 152:211–229
Davidson AL, Laghaeian SS, Mannering DE (1996) The maltose transport system of Escherichia coli displays positive cooperativity in ATP hydrolysis. J Biol Chem 271:4858–4863
Davidson AL, Dassa E, Orelle C, Chen J (2008) Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev 72:317–364
Dawson RJ, Locher KP (2006) Structure of a bacterial multidrug ABC transporter. Nature 443:180–185
Dawson RJ, Locher KP (2007) Structure of the multidrug ABC transporter Sav 1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett 581:935–938
Dawson RJ, Hollenstein K, Locher KP (2007) Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol Microbiol 65:250–257
de Veaux LC, Clevenson DS, Bradbeer C, Kadner RJ (1986) Identification of the btuCED polypeptides and evidence for their role in vitamin B12 transport in Escherichia coli. J Bacteriol 167:920–927
De Vos D, De Chial M, Cochez C, Jansen S, Tummler B, Meyer JM, Cornelis P (2001) Study of pyoverdine type and production by Pseudomonas aeruginosa isolated from cystic fibrosis patients: prevalence of type II pyoverdine isolates and accumulation of pyoverdine-negative mutations. Arch Microbiol 175:384–388
Debarbieux L, Wandersman C (2001) Folded HasA inhibits its own secretion through its ABC exporter. EMBO J 20:4657–4663
Delepelaire P, Wandersman C (1998) The SecB chaperone is involved in the secretion of the Serratia marcescens HasA protein through an ABC transporter. EMBO J 17:936–944
Detmers FJ, Lanfermeijer FC, Poolman B (2001) Peptides and ATP binding cassette peptide transporters. Res Microbiol 152:245–258
Dintilhac A, Alloing G, Granadel C, Claverys JP (1997) Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol 25:727–739
Doerrler WT, Reedy MC, Raetz CR (2001) An Escherichia coli mutant defective in lipid export. J Biol Chem 276:11461–11464
Doerrler WT, Gibbons HS, Raetz CR (2004) MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J Biol Chem 279:45102–45109
Doeven MK, Kok J, Poolman B (2005) Specificity and selectivity determinants of peptide transport in Lactococcus lactis and other microorganisms. Mol Microbiol 57:640–649
Duong F, Lazdunski A, Cami B, Murgier M (1992) Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene 121:47–54
Eitinger T, Rodionov DA, Grote M, Schneider E (2011) Canonical and ECF-type ATP-binding cassette importers in prokaryotes: diversity in modular organization and cellular functions. FEMS Microbiol Rev 35:3–67
Espinasse S, Gohar M, Lereclus D, Sanchis V (2002) An ABC transporter from Bacillus thuringiensis is essential for beta-exotoxin I production. J Bacteriol 184:5848–5854
Eswarappa SM, Panguluri KK, Hensel M, Chakravortty D (2008) The yejABEF operon of Salmonella confers resistance to antimicrobial peptides and contributes to its virulence. Microbiology 154:666–678
Faraldo-Gomez JD, Sansom MS (2003) Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol 4:105–116
Fetherston JD, Bertolino VJ, Perry RD (1999) YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol Microbiol 32:289–299
Fetzer AE, Werner AS, Hagstrom JW (1967) Pathologic features of pseudomonal pneumonia. Am Rev Respir Dis 96:1121–1130
Frey J, Meier R, Gygi D, Nicolet J (1991) Nucleotide sequence of the hemolysin I gene from Actinobacillus pleuropneumoniae. Infect Immun 59:3026–3032
Fronzes R, Christie PJ, Waksman G (2009) The structural biology of type IV secretion systems. Nat Rev Microbiol 7:703–714
Frosch M, Edwards U, Bousset K, Krausse B, Weisgerber C (1991) Evidence for a common molecular origin of the capsule gene loci in gram-negative bacteria expressing group II capsular polysaccharides. Mol Microbiol 5:1251–1263
Fuellen G, Spitzer M, Cullen P, Lorkowski S (2005) Correspondence of function and phylogeny of ABC proteins based on an automated analysis of 20 model protein data sets. Proteins 61:888–899
Gabbianelli R, Scotti R, Ammendola S, Petrarca P, Nicolini L, Battistoni A (2011) Role of ZnuABC and ZinT in Escherichia coli O157:H7 zinc acquisition and interaction with epithelial cells. BMC Microbiol 11:36
Garsin DA et al (2001) A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A 98:10892–10897
Ghanei H, Abeyrathne PD, Lam JS (2007) Biochemical characterization of MsbA from Pseudomonas aeruginosa. J Biol Chem 282:26939–26947
Gilmore MS, Segarra RA, Booth MC (1990) An HlyB-type function is required for expression of the Enterococcus faecalis hemolysin/bacteriocin. Infect Immun 58:3914–3923
Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A (1988) The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol 2:19–30
Glaser P et al (2001) Comparative genomics of Listeria species. Science 294:849–852
Goebel W, Hedgpeth J (1982) Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol 151:1290–1298
Gotz F (2002) Staphylococcus and biofilms. Mol Microbiol 43:1367–1378
Greenwald J, Hoegy F, Nader M, Journet L, Mislin GL, Graumann PL, Schalk IJ (2007) Real time fluorescent resonance energy transfer visualization of ferric pyoverdine uptake in Pseudomonas aeruginosa. A role for ferrous iron. J Biol Chem 282:2987–2995
Greller G, Horlacher R, DiRuggiero J, Boos W (1999) Molecular and biochemical analysis of MalK, the ATP-hydrolyzing subunit of the trehalose/maltose transport system of the hyperthermophilic archaeon Thermococcus litoralis. J Biol Chem 274:20259–20264
Griffin AS, West SA, Buckling A (2004) Cooperation and competition in pathogenic bacteria. Nature 430:1024–1027
Grigg JC, Vermeiren CL, Heinrichs DE, Murphy ME (2007) Heme coordination by Staphylococcus aureus IsdE. J Biol Chem 282:28815–28822
Groisman EA (1994) How bacteria resist killing by host-defense peptides. Trends in microbiology 2:444–449
Groisman EA, Parra-Lopez C, Salcedo M, Lipps CJ, Heffron F (1992) Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci U S A 89:11939–11943
Guilfoile PG, Hutchinson CR (1991) A bacterial analog of the mdr gene of mammalian tumor cells is present in Streptomyces peucetius, the producer of daunorubicin and doxorubicin. Proc Natl Acad Sci U S A 88:8553–8557
Gunasekera TS, Herre AH, Crowder MW (2009) Absence of ZnuABC-mediated zinc uptake affects virulence-associated phenotypes of uropathogenic Escherichia coli CFT073 under Zn(II)-depleted conditions. FEMS Microbiol Lett 300:36–41
Guzzo J, Murgier M, Filloux A, Lazdunski A (1990) Cloning of the Pseudomonas aeruginosa alkaline protease gene and secretion of the protease into the medium by Escherichia coli. J Bacteriol 172:942–948
Hanks TS, Liu M, McClure MJ, Lei B (2005) ABC transporter FtsABCD of Streptococcus pyogenes mediates uptake of ferric ferrichrome. BMC Microbiol 5:62
Hartel T, Klein M, Koedel U, Rohde M, Petruschka L, Hammerschmidt S (2011) Impact of glutamine transporters on pneumococcal fitness under infection-related conditions. Infect Immun 79:44–58
Hashimoto Y, Li N, Yokoyama H, Ezaki T (1993) Complete nucleotide sequence and molecular characterization of ViaB region encoding Vi antigen in Salmonella Typhimurium. J Bacteriol 175:4456–4465
Heck LW, Morihara K, Abrahamson DR (1986) Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect Immun 54:149–153
Hejazi A, Falkiner FR (1997) Serratia marcescens. J Med Microbiol 46:903–912
Henderson DP, Payne SM (1993) Cloning and characterization of the Vibrio cholerae genes encoding the utilization of iron from haemin and haemoglobin. Mol Microbiol 7:461–469
Henderson DP, Payne SM (1994) Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect Immun 62:5120–5125
Hess J, Wels W, Vogel M, Goebel W (1986) Nucleotide sequence of a plasmid-encoded haemolysin determinant and its comparison with a corresponding chromosomal haemolysin sequence. FEMS Microbiol Lett 34:1–11
Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8:67–113
Higgins MK, Bokma E, Koronakis E, Hughes C, Koronakis V (2004) Structure of the periplasmic component of a bacterial drug efflux pump. Proc Natl Acad Sci U S A 101:9994–9999
Hille M, Kies S, Gotz F, Peschel A (2001) Dual role of GdmH in producer immunity and secretion of the Staphylococcal antibiotics gallidermin and epidermin. Appl Environ Microbiol 67:1380–1383
Holland IB, Kenny B, Blight M (1990) Hemolysin secretion from Escherichia coli. Biochimie 72:131–141
Hollenstein K, Dawson RJP, Locher KP (2007a) Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol 17:412–418
Hollenstein K, Frei DC, Locher KP (2007b) Structure of an ABC transporter in complex with its binding protein. Nature 446:213–216
Hong YQ, Ghebrehiwet B (1992) Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin Immunol Immunopathol 62:133–138
Hooper NM (1994) Families of zinc metalloproteases. FEBS Lett 354:1–6
Hopfner KP, Tainer JA (2003) Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr Opin Struct Biol 13:249–255
Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA (2000) Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101:789–800
Horn C, Bremer E, Schmitt L (2005) Functional overexpression and in vitro re-association of OpuA, an osmotically regulated ABC-transport complex from Bacillus subtilis. FEBS Lett 579:5765–5768
Horn C, Sohn-Bosser L, Breed J, Welte W, Schmitt L, Bremer E (2006) Molecular determinants for substrate specificity of the ligand-binding protein OpuAC from Bacillus subtilis for the compatible solutes glycine betaine and proline betaine. J Mol Biol 357:592–606
Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ (2002) MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol 44:1269–1286
Horvat RT, Parmely MJ (1988) Pseudomonas aeruginosa alkaline protease degrades human gamma interferon and inhibits its bioactivity. Infect Immun 56:2925–2932
Huang X, Yan A, Zhang X, Xu Y (2006) Identification and characterization of a putative ABC transporter PltHIJKN required for pyoluteorin production in Pseudomonas sp. M18. Gene 376:68–78
Hung LW, Wang IX, Nikaido K, Liu PQ, Ames GF, Kim SH (1998) Crystal structure of the ATP-binding subunit of an ABC transporter. Nature 396:703–707
Hussain M, Heilmann C, Peters G, Herrmann M (2001) Teichoic acid enhances adhesion of Staphylococcus epidermidis to immobilized fibronectin. Microb Pathog 31:261–270
Huycke MM, Spiegel CA, Gilmore MS (1991) Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 35:1626–1634
Hvorup RN, Goetz BA, Niederer M, Hollenstein K, Perozo E, Locher KP (2007) Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD–BtuF. Science 317:1387–1390
Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR (2007) Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol 73:1107–1113
Ike Y, Hashimoto H, Clewell DB (1984) Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun 45:528–530
Ike Y, Hashimoto H, Clewell DB (1987) High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol 25:1524–1528
Ikeno S, Yamane Y, Ohishi Y, Kinoshita N, Hamada M, Tsuchiya KS, Hori M (2000) ABC transporter genes, kasKLM, responsible for self-resistance of a kasugamycin producer strain. J Antibiot (Tokyo) 53:373–384
Imperi F, Tiburzi F, Visca P (2009) Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 106:20440–20445
Janakiraman A, Slauch JM (2000) The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella Typhimurium. Mol Microbiol 35:1146–1155
Jansen R, Briaire J, Kamp EM, Gielkens AL, Smits MA (1993) Cloning and characterization of the Actinobacillus pleuropneumoniae-RTX-toxin III (ApxIII) gene. Infect Immun 61:947–954
Jenkinson HF (1994) Cell surface protein receptors in oral streptococci. FEMS Microbiol Lett 121:133–140
Johnson JM, Church GM (1999) Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J Mol Biol 287:695–715
Jones PM, George AM (1999) Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol Lett 179:187–202
Jones PM, George AM (2004) The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci 61:682–699
Kalscheuer R, Weinrick B, Veeraraghavan U, Besra GS, Jacobs WR Jr (2010) Trehalose-recycling ABC transporter LpqY–SugA–SugB–SugC is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 107:21761–21766
Kauffmann F (1947) The serology of the coli group. J Immunol 57:71–100
Kehres DG, Janakiraman A, Slauch JM, Maguire ME (2002) SitABCD is the alkaline Mn(2+) transporter of Salmonella enterica serovar Typhimurium. J Bacteriol 184:3159–3166
Keo T, Collins J, Kunwar P, Blaser MJ, Iovine NM (2011) Campylobacter capsule and lipooligosaccharide confer resistance to serum and cationic antimicrobials. Virulence 2:30–40
Kerr ID (2002) Structure and association of ATP-binding cassette transporter nucleotide-binding domains. Biochim Biophys Acta 1561:47–64
Kiran MD, Akiyoshi DE, Giacometti A, Cirioni O, Scalise G, Balaban N (2009) OpuC--an ABC transporter that is associated with Staphylococcus aureus pathogenesis. Int J Artif Organs 32:600–610.
Kitten T, Munro CL, Michalek SM, Macrina FL (2000) Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect Immun 68:4441–4451
Klein C, Entian KD (1994) Genes involved in self-protection against the lantibiotic subtilin produced by Bacillus subtilis ATCC 6633. Appl Environ Microbiol 60:2793–2801
Knapp S, Hacker J, Jarchau T, Goebel W (1986) Large, unstable inserts in the chromosome affect virulence properties of uropathogenic Escherichia coli O6 strain 536. J Bacteriol 168:22–30
Kolenbrander PE, Andersen RN, Baker RA, Jenkinson HF (1998) The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J Bacteriol 180:290–295
Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C (2000) Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914–919
Kroll JS, Loynds B, Brophy LN, Moxon ER (1990) The bex locus in encapsulated Haemophilus influenzae: a chromosomal region involved in capsule polysaccharide export. Mol Microbiol 4:1853–1862
Kroncke KD, Boulnois G, Roberts I, Bitter-Suermann D, Golecki JR, Jann B, Jann K (1990a) Expression of the Escherichia coli K5 capsular antigen: immunoelectron microscopic and biochemical studies with recombinant E. coli. J Bacteriol 172:1085–1091
Kroncke KD, Orskov I, Orskov F, Jann B, Jann K (1990b) Electron microscopic study of coexpression of adhesive protein capsules and polysaccharide capsules in Escherichia coli. Infect Immun 58:2710–2714
LaGier MJ, Threadgill DS (2008) Identification of novel genes in the oral pathogen Campylobacter rectus. Oral Microbiol Immunol 23:406–412
Lawrence MC, Pilling PA, Epa VC, Berry AM, Ogunniyi AD, Paton JC (1998) The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6:1553–1561
Lazarevic V, Karamata D (1995) The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol Microbiol 16:345–355
Lee EW, Huda MN, Kuroda T, Mizushima T, Tsuchiya T (2003) EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob Agents Chemother 47:3733–3738
Lei B, Liu M, Voyich JM, Prater CI, Kala SV, DeLeo FR, Musser JM (2003) Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes. Infect Immun 71:5962–5969
Letoffe S, Ghigo JM, Wandersman C (1994a) Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc Natl Acad Sci U S A 91:9876–9880
Letoffe S, Ghigo JM, Wandersman C (1994b) Secretion of the Serratia marcescens HasA protein by an ABC transporter. J Bacteriol 176:5372–5377
Liao CH, McCallus DE (1998) Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091. Appl Environ Microbiol 64:914–921
Lim KH et al (2008) Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect Immun 76:3569–3576
Lin W et al (1999) Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci U S A 96:1071–1076
Linhartova I et al (2010) RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol Rev 34:1076–1112
Liu CE, Liu PQ, Ames GF (1997) Characterization of the adenosine triphosphatase activity of the periplasmic histidine permease, a traffic ATPase (ABC transporter). J Biol Chem 272:21883–21891
Locher KP (2009) Review. Structure and mechanism of ATP-binding cassette transporters. Philos Trans R Soc Lond B Biol Sci 364:239–245
Locher KP, Lee AT, Rees DC (2002) The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 296:1091–1098
Loisel E et al (2008) AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J Mol Biol 381:594–606
Loomes LM, Kerr MA, Senior BW (1993) The cleavage of immunoglobulin G in vitro and in vivo by a proteinase secreted by the urinary tract pathogen Proteus mirabilis. J Med Microbiol 39:225–232
Lu G, Westbrooks JM, Davidson AL, Chen J (2005) ATP hydrolysis is required to reset the ATP-binding cassette dimer into the resting-state conformation. Proc Natl Acad Sci U S A 102:17969–17974
Lubelski J, Mazurkiewicz P, van Merkerk R, Konings WN, Driessen AJ (2004) ydaG and ydbA of Lactococcus lactis encode a heterodimeric ATP-binding cassette-type multidrug transporter. J Biol Chem 279:34449–34455
Lubelski J, de Jong A, van Merkerk R, Agustiandari H, Kuipers OP, Kok J, Driessen AJ (2006a) LmrCD is a major multidrug resistance transporter in Lactococcus lactis. Mol Microbiol 61:771–781
Lubelski J, van Merkerk R, Konings WN, Driessen AJ (2006b) Nucleotide-binding sites of the heterodimeric LmrCD ABC-multidrug transporter of Lactococcus lactis are asymmetric. Biochemistry 45:648–656
Ludwig, A, and Goebel, W (1999) The family of the multigenic encoded RTX toxin. In: JE Alouf, and JH Freer (eds) The comprehensive sourcebook of bacterial protein toxins. Academic, London, pp 330–348
Mackedonski VV, Nikolaev N, Sebesta K, Hadjiolov AA (1972) Inhibition of ribonucleic acid biosynthesis in mice liver by the exotoxin of Bacillus thuringiensis. Biochim Biophys Acta 272:56–66
Maier E, Reinhard N, Benz R, Frey J (1996) Channel-forming activity and channel size of the RTX toxins ApxI, ApxII, and ApxIII of Actinobacillus pleuropneumoniae. Infect Immun 64:4415–4423
Mason KM, Bruggeman ME, Munson RS, Bakaletz LO (2006) The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol 62:1357–1372
Massonet C, Pintens V, Merckx R, Anne J, Lammertyn E, Van Eldere J (2006) Effect of iron on the expression of sirR and sitABC in biofilm-associated Staphylococcus epidermidis. BMC Microbiol 6:103
Matsumoto-Nakano M, Kuramitsu HK (2006) Role of bacteriocin immunity proteins in the antimicrobial sensitivity of Streptococcus mutans. J Bacteriol 188:8095–8102
Matsuo T, Chen J, Minato Y, Ogawa W, Mizushima T, Kuroda T, Tsuchiya T (2008) SmdAB, a heterodimeric ABC-Type multidrug efflux pump, in Serratia marcescens. J Bacteriol 190:648–654
McAllister LJ, Tseng HJ, Ogunniyi AD, Jennings MP, McEwan AG, Paton JC (2004) Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol Microbiol 53:889–901
McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC (2011) A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7:e1002357
McNulty C, Thompson J, Barrett B, Lord L, Andersen C, Roberts IS (2006) The cell surface expression of group 2 capsular polysaccharides in Escherichia coli: the role of KpsD, RhsA and a multi-protein complex at the pole of the cell. Mol Microbiol 59:907–922
Mendez C, Salas JA (2001) The role of ABC transporters in antibiotic-producing organisms: drug secretion and resistance mechanisms. Res Microbiol 152:341–350
Menendez N, Brana AF, Salas JA, Mendez C (2007) Involvement of a chromomycin ABC transporter system in secretion of a deacetylated precursor during chromomycin biosynthesis. Microbiology 153:3061–3070
Menestrina G, Moser C, Pellet S, Welch R (1994) Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology 87:249–267
Menestrina G, Dalla Serra M, Pederzolli C, Bregante M, Gambale F (1995a) Bacterial hemolysins and leukotoxins affect target cells by forming large exogenous pores into their plasma membrane: Escherichia coli hemolysin A as a case example. Biosci Rep 15:543–551
Menestrina G, Ropele M, Dalla Serra M, Pederzolli C, Hugo F, Pellet S, Welch RA (1995b) Binding of antibodies to functional epitopes on the pore formed by Escherichia coli hemolysin in cells and model membranes. Biochim Biophys Acta 1238:72–80
Meyer C et al (1995) Nucleotide sequence of the lantibiotic Pep5 biosynthetic gene cluster and functional analysis of PepP and PepC. Evidence for a role of PepC in thioether formation. Eur J Biochem 232:478–489
Miyamoto M, Maeda H, Kitanaka M, Kokeguchi S, Takashiba S, Murayama Y (1998) The S-layer protein from Campylobacter rectus: sequence determination and function of the recombinant protein. FEMS Microbiol Lett 166:275–281
Miyoshi S, Shinoda S (2000) Microbial metalloproteases and pathogenesis. Microbes Infect 2:91–98
Moeck GS, Coulton JW (1998) TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol Microbiol 28:675–681
Moody JE, Thomas PJ (2005) Nucleotide binding domain interactions during the mechanochemical reaction cycle of ATP-binding cassette transporters. J Bioenerg Biomembr 37:475–479
Morrissey JA, Cockayne A, Hill PJ, Williams P (2000) Molecular cloning and analysis of a putative siderophore ABC transporter from Staphylococcus aureus. Infect Immun 68:6281–6288
Moxon ER, Vaughn KA (1981) The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis 143:517–524
Netz DJ, Sahl HG, Marcelino R, dos Santos Nascimento J, de Oliveira SS, Soares MB, do Carmo deFreire Bastos M (2001) Molecular characterisation of aureocin A70, a multi-peptide bacteriocin isolated from Staphylococcus aureus. J Mol Biol 311:939–949
Novikova M, Metlitskaya A, Datsenko K, Kazakov T, Kazakov A, Wanner B, Severinov K (2007) The Escherichia coli Yej transporter is required for the uptake of translation inhibitor microcin C. J Bacteriol 189:8361–8365
Nygaard TK et al (2006) The mechanism of direct heme transfer from the streptococcal cell surface protein Shp to HtsA of the HtsABC transporter. J Biol Chem 281:20761–20771
Occhino DA, Wyckoff EE, Henderson DP, Wrona TJ, Payne SM (1998) Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol Microbiol 29:1493–1507
Ochsner UA, Johnson Z, Vasil ML (2000) Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology 146:185–198
Okuda K et al (1997) Role for the S-layer of Campylobacter rectus ATCC33238 in complement mediated killing and phagocytic killing by leukocytes from guinea pig and human peripheral blood. Oral Dis 3:113–120
Olano C, Rodriguez AM, Mendez C, Salas JA (1995) A second ABC transporter is involved in oleandomycin resistance and its secretion by Streptomyces antibioticus. Mol Microbiol 16:333–343
Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J (2007) Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450:515–521
Otto M (2009) Bacterial sensing of antimicrobial peptides. Contrib Microbiol 16:136–149
Otto M, Gotz F (2001) ABC transporters of staphylococci. Res Microbiol 152:351–356
Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T (2003) The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol 185:5967–5975
Papadelli M, Karsioti A, Anastasiou R, Georgalaki M, Tsakalidou E (2007) Characterization of the gene cluster involved in the biosynthesis of macedocin, the lantibiotic produced by Streptococcus macedonicus. FEMS Microbiol Lett 272:75–82
Papp-Wallace KM, Maguire ME (2006) Manganese transport and the role of manganese in virulence. Annu Rev Microbiol 60:187–209
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195
Parra-Lopez C, Baer MT, Groisman EA (1993) Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella Typhimurium. EMBO J 12:4053–4062
Paulsen IT, Park JH, Choi PS, Saier MH Jr (1997) A family of gram-negative bacterial outer membrane factors that function in the export of proteins, carbohydrates, drugs and heavy metals from gram-negative bacteria. FEMS Microbiol Lett 156:1–8
Pavelka MS Jr, Wright LF, Silver RP (1991) Identification of two genes, kpsM and kpsT, in region 3 of the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol 173:4603–4610
Pavelka MS Jr, Hayes SF, Silver RP (1994) Characterization of KpsT, the ATP-binding component of the ABC-transporter involved with the export of capsular polysialic acid in Escherichia coli K1. J Biol Chem 269:20149–20158
Pazzani C, Rosenow C, Boulnois GJ, Bronner D, Jann K, Roberts IS (1993) Molecular analysis of region 1 of the Escherichia coli K5 antigen gene cluster: a region encoding proteins involved in cell surface expression of capsular polysaccharide. J Bacteriol 175:5978–5983
Perry RD, Mier I Jr, Fetherston JD (2007) Roles of the Yfe and Feo transporters of Yersinia pestis in iron uptake and intracellular growth. Biometals 20:699–703
Peschel A, Gotz F (1996) Analysis of the Staphylococcus epidermidis genes epiF, -E, and -G involved in epidermin immunity. J Bacteriol 178:531–536
Petrarca P, Ammendola S, Pasquali P, Battistoni A (2010) The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J Bacteriol 192:1553–1564
Pigeon RP, Silver RP (1994) Topological and mutational analysis of KpsM, the hydrophobic component of the ABC-transporter involved in the export of polysialic acid in Escherichia coli K1. Mol Microbiol 14:871–881
Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC (2007) An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science 315:373–377
Pluschke G, Mayden J, Achtman M, Levine RP (1983a) Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun 42:907–913
Pluschke G, Mercer A, Kusecek B, Pohl A, Achtman M (1983b) Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun 39:599–608
Poelarends GJ, Mazurkiewicz P, Putman M, Cool RH, Veen HW, Konings WN (2000) An ABC-type multidrug transporter of Lactococcus lactis possesses an exceptionally broad substrate specificity. Drug Resist Updat 3:330–334
Poelarends GJ, Mazurkiewicz P, Konings WN (2002) Multidrug transporters and antibiotic resistance in Lactococcus lactis. Biochim Biophys Acta 1555:1–7
Poltorak A et al (1998) Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis 24:340–355
Postle K (1990) TonB and the gram-negative dilemma. Mol Microbiol 4:2019–2025
Price CT, Bukka A, Cynamon M, Graham JE (2008) Glycine betaine uptake by the ProXVWZ ABC transporter contributes to the ability of Mycobacterium tuberculosis to initiate growth in human macrophages. J Bacteriol 190:3955–3961
Raetz CR, Reynolds CM, Trent MS, Bishop RE (2007) Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem 76:295–329
Ravaud S et al (2006) The ABC transporter BmrA from Bacillus subtilis is a functional dimer when in a detergent-solubilized state. Biochem J 395:345–353
Rees DC, Johnson E, Lewinson O (2009) ABC transporters: the power to change. Nat Rev Mol Cell Biol 10:218–227
Reuter G, Janvilisri T, Venter H, Shahi S, Balakrishnan L, van Veen HW (2003) The ATP binding cassette multidrug transporter LmrA and lipid transporter MsbA have overlapping substrate specificities. J Biol Chem 278:35193–35198
Rince A, Dufour A, Uguen P, Le Pennec JP, Haras D (1997) Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl Environ Microbiol 63:4252–4260
Rodriguez GM, Smith I (2006) Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J Bacteriol 188:424–430
Rodriguez AM, Olano C, Vilches C, Mendez C, Salas JA (1993) Streptomyces antibioticus contains at least three oleandomycin-resistance determinants, one of which shows similarity with proteins of the ABC-transporter superfamily. Mol Microbiol 8:571–582
Rosinha GM et al (2002) Identification and characterization of a Brucella abortus ATP-binding cassette transporter homolog to Rhizobium meliloti ExsA and its role in virulence and protection in mice. Infect Immun 70:5036–5044
Rossi MS, Fetherston JD, Letoffe S, Carniel E, Perry RD, Ghigo JM (2001) Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis. Infect Immun 69:6707–6717
Ryndak MB, Wang S, Smith I, Rodriguez GM (2010) The Mycobacterium tuberculosis high-affinity iron importer, IrtA, contains an FAD-binding domain. J Bacteriol 192:861–869
Sabri M et al (2008) Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain chi7122. Infect Immun 76:601–611
Sabri M, Houle S, Dozois CM (2009) Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect Immun 77:1155–1164
Saier MH Jr, Tran CV, Barabote RD (2006) TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res 34:D181–186
Saier MH, Ma CH, Rodgers L, Tamang DG, Yen MR (2008) Protein secretion and membrane insertion systems in bacteria and eukaryotic organelles. Adv Appl Microbiol 65:141–197
Sakamoto K, Margolles A, van Veen HW, Konings WN (2001) Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J Bacteriol 183:5371–5375
Sanders JD, Cope LD, Hansen EJ (1994) Identification of a locus involved in the utilization of iron by Haemophilus influenzae. Infect Immun 62:4515–4525
Schalk IJ, Abdallah MA, Pattus F (2002) Recycling of pyoverdin on the FpvA receptor after ferric pyoverdin uptake and dissociation in Pseudomonas aeruginosa. Biochemistry 41:1663–1671
Schaller A et al (1999) Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology 145(Pt 8):2105–2116
Schirner K, Stone LK, Walker S (2011) ABC transporters required for export of wall teichoic acids do not discriminate between different main chain polymers. ACS Chem Biol 6:407–412
Schlievert PM, Dunny GM, Stoehr JA, Assimacopoulos AP (1997) Aggregation and binding substances enhance pathogenicity in a rabbit model of Enterococcus faecalis endocarditis. Adv Exp Med Biol 418:789–791
Schmidt H, Beutin L, Karch H (1995) Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun 63:1055–1061
Schmidt H, Maier E, Karch H, Benz R (1996) Pore-forming properties of the plasmid-encoded hemolysin of enterohemorrhagic Escherichia coli O157:H7. Eur J Biochem 241:594–601
Schneider E, Hunke S (1998) ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol Rev 22:1–20
Schoner B et al (1992) Sequence similarity between macrolide-resistance determinants and ATP-binding transport proteins. Gene 115:93–96
Schreur PJ, Rebel JM, Smits MA, van Putten JP, Smith HE (2011) TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol 193:5073–5080
Schulein R, Gentschev I, Mollenkopf HJ, Goebel W (1992) A topological model for the haemolysin translocator protein HlyD. Mol Gen Genet 234:155–163
Sebesta K, Horska K (1970) Mechanism of inhibition of DNA-dependent RNA polymerase by exotoxin of Bacillus thuringiensis. Biochim Biophys Acta 209:357–376
Shea CM, McIntosh MA (1991) Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol 5:1415–1428
Shibuya Y, Yamamoto T, Morimoto T, Nishino N, Kambara T, Okabe H (1991) Pseudomonas aeruginosa alkaline proteinase might share a biological function with plasmin. Biochim Biophys Acta 1077:316–324
Shitan N et al (2003) Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci U S A 100:751–756
Shouldice SR, Skene RJ, Dougan DR, Snell G, McRee DE, Schryvers AB, Tari LW (2004) Structural basis for iron binding and release by a novel class of periplasmic iron-binding proteins found in gram-negative pathogens. J Bacteriol 186:3903–3910
Siarheyeva A, Sharom FJ (2009) The ABC transporter MsbA interacts with lipid A and amphipathic drugs at different sites. Biochem J 419:317–328
Siegers K, Entian KD (1995) Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Appl Environ Microbiol 61:1082–1089
Silver RP, Aaronson W, Vann WF (1987) Translocation of capsular polysaccharides in pathogenic strains of Escherichia coli requires a 60-kilodalton periplasmic protein. J Bacteriol 169:5489–5495
Sleator RD, Wouters J, Gahan CG, Abee T, Hill C (2001) Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl Environ Microbiol 67:2692–2698
Smith AN, Boulnois GJ, Roberts IS (1990) Molecular analysis of the Escherichia coli K5 kps locus: identification and characterization of an inner-membrane capsular polysaccharide transport system. Mol Microbiol 4:1863–1869
Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF (2002) ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell 10:139–149
Solbiati JO, Ciaccio M, Farias RN, Gonzalez-Pastor JE, Moreno F, Salomon RA (1999) Sequence analysis of the four plasmid genes required to produce the circular peptide antibiotic microcin J25. J Bacteriol 181:2659–2662
Speziali CD, Dale SE, Henderson JA, Vines ED, Heinrichs DE (2006) Requirement of Staphylococcus aureus ATP-binding cassette-ATPase FhuC for iron-restricted growth and evidence that it functions with more than one iron transporter. J Bacteriol 188:2048–2055
Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, van der Ley P (1998) Meningitis bacterium is viable without endotoxin. Nature 392:449–450
Steinfels E et al (2004) Characterization of YvcC (BmrA), a multidrug ABC transporter constitutively expressed in Bacillus subtilis. Biochemistry 43:7491–7502
Stocker W, Bode W (1995) Structural features of a superfamily of zinc-endopeptidases: the metzincins. Curr Opin Struct Biol 5:383–390
Stocker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, McKay DB, Bode W (1995) The metzincins—topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci 4:823–840
Stumpe S, Bakker EP (1997) Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch Microbiol 167:126–136
Sun X, Ge R, Zhang D, Sun H, He QY (2010) Iron-containing lipoprotein SiaA in SiaABC, the primary heme transporter of Streptococcus pyogenes. J Biol Inorg Chem 15:1265–1273
Sutcliffe IC, Russell RR (1995) Lipoproteins of gram-positive bacteria. J Bacteriol 177:1123–1128
Taboy CH, Vaughan KG, Mietzner TA, Aisen P, Crumbliss AL (2001) Fe3+ coordination and redox properties of a bacterial transferrin. J Biol Chem 276:2719–2724
Tamura J et al (1999) Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol 116:28–32
Tefsen B, Bos MP, Beckers F, Tommassen J, de Cock H (2005) MsbA is not required for phospholipid transport in Neisseria meningitidis. J Biol Chem 280:35961–35966
Terasaka K et al (2005) PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17:2922–2939
Thanabalu T, Koronakis E, Hughes C, Koronakis V (1998) Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J 17:6487–6496
Thompson SA (2002) Campylobacter surface-layers (S-layers) and immune evasion. Ann Periodontol 7:43–53
Thompson SA, Shedd OL, Ray KC, Beins MH, Jorgensen JP, Blaser MJ (1998) Campylobacter fetus surface layer proteins are transported by a type I secretion system. J Bacteriol 180:6450–6458
Tong Y, Guo M (2009) Bacterial heme-transport proteins and their heme-coordination modes. Arch Biochem Biophys 481:1–15
Tseng HJ, McEwan AG, Paton JC, Jennings MP (2002) Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun 70:1635–1639
van Der Heide T, Poolman B (2000) Glycine betaine transport in Lactococcus lactis is osmotically regulated at the level of expression and translocation activity. J Bacteriol 182:203–206
van der Heide T, Poolman B (2002) ABC transporters: one, two or four extracytoplasmic substrate-binding sites? EMBO Rep 3:938–943
van Veen HW et al (1996) Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci U S A 93:10668–10672
Velamakanni S, Yao Y, Gutmann DA, van Veen HW (2008) Multidrug transport by the ABC transporter Sav 1866 from Staphylococcus aureus. Biochemistry 47:9300–9308
Velamakanni S et al (2009) A multidrug ABC transporter with a taste for salt. PLoS One 4:e6137
Walker KE, Moghaddame-Jafari S, Lockatell CV, Johnson D, Belas R (1999) ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol Microbiol 32:825–836
Wandersman C, Delepelaire P (2004) Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58:611–647
Wang CC, Newton A (1971) An additional step in the transport of iron defined by the tonB locus of Escherichia coli. J Biol Chem 246:2147–2151
Wang RC, Seror SJ, Blight M, Pratt JM, Broome-Smith JK, Holland IB (1991) Analysis of the membrane organization of an Escherichia coli protein translocator, HlyB, a member of a large family of prokaryote and eukaryote surface transport proteins. J Mol Biol 217:441–454
Wang B, Kraig E, Kolodrubetz D (1998) A new member of the S-layer protein family: characterization of the crs gene from Campylobacter rectus. Infect Immun 66:1521–1526
Wassif C, Cheek D, Belas R (1995) Molecular analysis of a metalloprotease from Proteus mirabilis. J Bacteriol 177:5790–5798
Weidenmaier C, Peschel A, Xiong YQ, Kristian SA, Dietz K, Yeaman MR, Bayer AS (2005) Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J Infect Dis 191:1771–1777
Welch RA, Dellinger EP, Minshew B, Falkow S (1981) Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature 294:665–667
Wemekamp-Kamphuis HH, Wouters JA, Sleator RD, Gahan CG, Hill C, Abee T (2002) Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl Environ Microbiol 68:4710–4716
Weston BF, Brenot A, Caparon MG (2009) The metal homeostasis protein, Lsp, of Streptococcus pyogenes is necessary for acquisition of zinc and virulence. Infect Immun 77:2840–2848
Whitfield C (2006) Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75:39–68
Whitfield C, Richards JC, Perry MB, Clarke BR, MacLean LL (1991) Expression of two structurally distinct d-galactan O antigens in the lipopolysaccharide of Klebsiella pneumoniae serotype O1. J Bacteriol 173:1420–1431
Wickner W, Schekman R (2005) Protein translocation across biological membranes. Science 310:1452–1456
Woods RG, Burger M, Beven CA, Beacham IR (2001) The aprX-lipA operon of Pseudomonas fluorescens B52: a molecular analysis of metalloprotease and lipase production. Microbiology 147:345–354
Wyckoff EE, Valle AM, Smith SL, Payne SM (1999) A multifunctional ATP-binding cassette transporter system from Vibrio cholerae transports vibriobactin and enterobactin. J Bacteriol 181:7588–7596
Yang CC, Konisky J (1984) Colicin V-treated Escherichia coli does not generate membrane potential. J Bacteriol 158:757–759
Zaharik ML et al (2004) The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun 72:5522–5525
Zaidi AH, Bakkes PJ, Lubelski J, Agustiandari H, Kuipers OP, Driessen AJ (2008) The ABC-type multidrug resistance transporter LmrCD is responsible for an extrusion-based mechanism of bile acid resistance in Lactococcus lactis. J Bacteriol 190:7357–7366
Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L (2005a) H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J 24:1901–1910
Zaitseva J, Jenewein S, Wiedenmann A, Benabdelhak H, Holland IB, Schmitt L (2005b) Functional characterization and ATP-induced dimerization of the isolated ABC-domain of the haemolysin B transporter. Biochemistry 44:9680–9690
Zhang L, Al-Hendy A, Toivanen P, Skurnik M (1993) Genetic organization and sequence of the rfb gene cluster of Yersinia enterocolitica serotype O:3: similarities to the dTDP-l-rhamnose biosynthesis pathway of Salmonella and to the bacterial polysaccharide transport systems. Mol Microbiol 9:309–321
Zhong X, Kolter R, Tai PC (1996) Processing of colicin V-1, a secretable marker protein of a bacterial ATP binding cassette export system, requires membrane integrity, energy, and cytosolic factors. J Biol Chem 271:28057–28063
Zolghadr B, Weber S, Szabo Z, Driessen AJ, Albers SV (2007) Identification of a system required for the functional surface localization of sugar binding proteins with class III signal peptides in Sulfolobus solfataricus. Mol Microbiol 64:795–806
Zutz A, Hoffmann J, Hellmich UA, Glaubitz C, Ludwig B, Brutschy B, Tampe R (2011) Asymmetric ATP hydrolysis cycle of the heterodimeric multidrug ABC transport complex TmrAB from Thermus thermophilus. J Biol Chem 286:7104–7115
Acknowledgements
MPW is supported by the Channel 7 Children’s Research Foundation (Grant #103) and VGL is supported by an Australian Postgraduate Award and Postgraduate Stipend from the Australian Cystic Fibrosis Research Foundation. We thank James C. Paton for critical insight and discussions.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: David Robinson
Rights and permissions
About this article
Cite this article
Lewis, V.G., Ween, M.P. & McDevitt, C.A. The role of ATP-binding cassette transporters in bacterial pathogenicity. Protoplasma 249, 919–942 (2012). https://doi.org/10.1007/s00709-011-0360-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0360-8