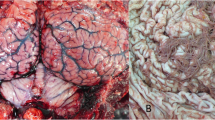
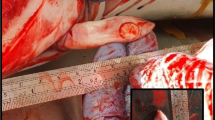
Abstract
We have detected a high incidence of lymphomas in a colony of GASH:Sal Syrian golden hamsters (Mesocricetus auratus). This strain is characterised by its ability to present convulsive crises of audiogenic origin. Almost 16 % (90 males and 60 females) of the 975 animals were affected during a 5-year period by the development of a progressing lymphoid tumour and exhibited similar clinical profiles characterised by lethargy, anorexia, evident abdominal distension, and a rapid disease progression resulting in mortality within 1 to 2 weeks. A TaqMan® probe-based real-time PCR analysis of genomic DNA from different tissue samples of the affected animals revealed the presence of a DNA sequence encoding the hamster polyomavirus (HaPyV) VP1 capsid protein. Additionally, immunohistochemical analysis using HaPyV-VP1-specific monoclonal antibodies confirmed the presence of viral proteins in all hamster tumour tissues analysed within the colony. An indirect ELISA and western blot analysis confirmed the presence of antibodies against the VP1 capsid protein in sera, not only from affected and non-affected GASH:Sal hamsters but also from control hamsters from the same breeding area. The HaPyV genome that accumulated in tumour tissues typically contained deletions affecting the noncoding regulatory region and adjacent sequences coding for the N-terminal part of the capsid protein VP2.







Similar content being viewed by others
References
Ambrose KR, Coggin JJ (1975) An epizootic in hamsters of lymphomas of undetermined origin and mode of transmission. J Natl Cancer Inst 54:877–880
Barthold SW, Bhatt PN, Johnson EA (1987) Further evidence for the papovavirus as the probable etiology of transmissible lymphoma of Syrian hamsters. Lab Anim Sci 37:283–288
Burchfield SR, Woods SC, Elich MS (1978) Effects of cold stress on tumor growth. Physiol behav 21:537–540
Coggin JH Jr, Oakes JE, Huebner RJ, Gilden R (1981) Unusual filterable oncogenic agent isolated from horizontally transmitted Syrian hamster lymphomas. Nature 290:336–338
Fermand JP, Mitjavila MT, Le Couedic JP, Tsapis A, Berger R, Modigliani R, Seligmann M, Brouet JC, Vainchenker W (1993) Role of granulocyte-macrophage colony-stimulating factor, interleukin-3 and interleukin-5 in the eosinophilia associated with T cell lymphoma. Brit J haematol 83:359–364
Foster AP, Brown PJ, Jandrig B, Grosch A, Voronkova T, Scherneck S, Ulrich R (2002) Polyomavirus infection in hamsters and trichoepitheliomas/cutaneous adnexal tumours. Vet Rec 151:13–17
Freeman JL, Perry GH, Feuk L, Redon R, McCarroll SA, Altshuler DM, Aburatani H, Jones KW, Tyler-Smith C, Hurles ME, Carter NP, Scherer SW, Lee C (2006) Copy number variation: new insights in genome diversity. Genome res 16:949–961
Gabor LJ, Canfield PJ, Malik R (2000) Haematological and biochemical findings in cats in Australia with lymphosarcoma. Aust Vet J 78:456–461
Gasparini G, Pozza F, Meli S, Reitano M, Santini G, Bevilacqua P (1991) Breast cancer cell kinetics: immunocytochemical determination of growth fractions by monoclonal antibody Ki-67 and correlation with flow cytometric S-phase and with some features of tumor aggressiveness. Anticancer Res 11:2015–2021
Graffi A, Bender E, Schramm T, Kuhn W, Schneiders F (1969) Induction of transmissible lymphomas in Syrian hamsters by application of DNA from viral hamster papovavirus-induced tumors and by cell-free filtrates from human tumors. Proc Nat Acad Sci USA 64:1172–1175
Graffi A, Schramm T, Graffi I, Bierwolf D, Bender E (1968) Virus-associated skin tumors of the Syrian hamster: preliminary note. J Nat Cancer Inst 40:867–873
Guidotti LG (2003) Pathogenesis of viral hepatitis. J Biol Reg Homeos Ag 17:115–119
Higgins ME, Claremont M, Major JE, Sander C, Lash AE (2007) Cancer-Genes: a gene selection resource for cancer genome projects. Nucleic Acids Res 35:D721–D726
Leoncini L, Raphael M, Stein H, Harris NL, Jaffe ES, Kluin PM (2008) Burkitt lymphoma. In: Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (eds) World Health Organisation classification of tumours of haematopoietic and lymphoid tissues. IARC Press, Lyon, pp 262–264
McMartin DN (1979) Morphologic lesions in aging Syrian hamsters. J Gerontol 34:502–511
Muñoz LJ, López DE (2005) Establecimiento y caracterización de una línea de hámsters sirios propensos a padecer convulsiones audiógenas, Salamanca
Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B (2002) Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42
Percy DH, Barthold SW (1993) Pathology of laboratory rodents and rabbits. Iowa State University Press, Ames
Prokoph H, Jandrig B, Arnold W, Scherneck S (1997) Generation of lymphoma-type variant hamster polyomavirus genomes in hamsters susceptible to lymphoma induction. Arch Virol 142:53–63
Scherneck S, Ulrich R, Feunteun J (2001) The hamster polyomavirus–a brief review of recent knowledge. Virus genes 22:93–101
Shlien A, Malkin D (2009) Copy number variations and cancer. Genome Med 1:62
Simmons JH, Riley LK, Franklin CL, Besch-Williford CL (2001) Hamster polyomavirus infection in a pet Syrian hamster (Mesocricetus auratus). Vet Pathol 38:441–446
Strandberg JD (1987) Neoplastic diseases. In: Van Hoosier GL, McPherson CW (eds) Laboratory hamsters. Academic Press, Orlando, pp 157–168
Strauli P, Mettler J (1982) Tumors of the hamsters. In: Turusov VS (ed) Pathology of Tumors in Laboratory Animals. Lyon, pp 343–370
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2008) World Health Organisation classification of tumors of hematopoietic and lymphoid tissues, 4th edn. IARC Press, Lyon
Ulrich R, Sommerfeld K, Schroder A, Prokoph H, Arnold W, Kruger DH, Scherneck S (1996) Hamster polyomavirus-encoded proteins: gene cloning, heterologous expression and immunoreactivity. Virus Genes 12:265–274
Vegas O, Fano E, Brain PF, Alonso A, Azpiroz A (2006) Social stress, coping strategies and tumor development in male mice: behavioral, neuroendocrine and immunological implications. Psychoneuroendocrinology 31:69–79
Voronkova T, Grosch A, Kazaks A, Ose V, Skrastina D, Sasnauskas K, Jandrig B, Arnold W, Scherneck S, Pumpens P, Ulrich R (2002) Chimeric bacteriophage fr virus-like particles harboring the immunodominant C-terminal region of hamster polyomavirus VP1 induce a strong VP1-specific antibody response in rabbits and mice. Viral Immunol 15:627–643
Zvirbliene A, Samonskyte L, Gedvilaite A, Voronkova T, Ulrich R, Sasnauskas K (2006) Generation of monoclonal antibodies of desired specificity using chimeric polyomavirus-derived virus-like particles. J Immunol Methods 311:57–70
Acknowledgments
The authors are very grateful to Christine Fast for helpful discussion concerning histopathology, and to Manuel Sánchez Martín and Javier Herrero Turrion for their help in the molecular biology experiments. We acknowledge the support by the European Regional Development Fund 2010/0314/2DP/2.1.1.1.0/10/APIA/VIAA/052 (Tatyana Voronkova) and Spanish JCyL (#SA023A12-2) and USP-USAL 2011-6 (D.E. López) and Fundación Mutua Madrileña AP22592008 (L.J. Muñoz).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñoz, L.J., Ludeña, D., Gedvilaite, A. et al. Lymphoma outbreak in a GASH:Sal hamster colony. Arch Virol 158, 2255–2265 (2013). https://doi.org/10.1007/s00705-013-1737-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-013-1737-0