Abstract
Purpose
Oncology clinical trials are necessary for the improvement of patient care as they have the ability to confirm the efficacy and safety of novel cancer treatments and in so doing, contribute to a solid evidence base on which practitioners and patients can make informed treatment decisions. However, only 3–5 % of adult cancer patients enroll in clinical trials. Lack of participation compromises the success of clinical trials and squanders an opportunity for improving patient outcomes. This literature review summarizes the factors and contexts that influence cancer patient decision making related to clinical trial participation.
Methods
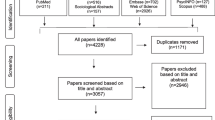
An integrative review was undertaken within PubMed, CINAHL, and EMBASE databases for articles written between 1995 and 2012 and archived under relevant keywords. Articles selected were data-based, written in English, and limited to adult cancer patients.
Results
In the 51 articles reviewed, three main types of factors were identified that influence cancer patients’ decision making about participation in clinical trials: personal, social, and system factors. Subthemes included patients’ trust in their physician and the research process, undue influence within the patient-physician relationship, and systemic social inequalities. How these factors interact and influence patients’ decision-making process and relational autonomy, however, is insufficiently understood.
Conclusions
Future research is needed to further elucidate the sociopolitical barriers and facilitators of clinical trial participation and to enhance ethical practice within clinical trial enrolment. This research will inform targeted education and support interventions to foster patients’ relational autonomy in the decision-making process and potentially improve clinical trial participation rates.
Similar content being viewed by others
Notes
It is not clear in many of these studies whether physicians informing patients about CTs were treating physicians or physician-investigators. This difference may have important implications for patients’ relational autonomy. For example, the dual role of physician-investigators may impact the trust patients have in these physicians and the way in which information was provided, since physician-investigators could have secondary interests that conflict with the patient’s best interest.
Clinical equipoise means there is genuine uncertainty in the expert medical community about whether a treatment will be beneficial [82].
References
Wasan AD, Taubenberger SP, Robinson WM (2009) Reasons for participation in pain research: can they indicate a lack of informed consent? Pain Med 10:111–119
Lee SJ, Lenert L, Weisman S, Kavanaugh A (2005) Factors affecting rheumatoid arthritis patients’ decisions to participate in clinical trials. J Rheumatol 32:2317–2325
Unson CG, Dunbar N, Curry L, Kenyon L, Prestwood K (2001) The effects of knowledge, attitudes, and significant others on decisions to enroll in a clinical trial on osteoporosis: implications for recruitment of older African-American women. J Natl Med Assoc 93:392–401, discussion 402–4
Burgess LJ, Sulzer NU, Hoosain F, Leverton N, Bliganut S, Emanuel S (2009) Patients’ motivations for participating in cardiovascular clinical trials: a local perspective. Cardiovasc J Afr 20:220–223
Glover M, Kira A, Johnston V, Walker N, Thomas D, Change A, Bullen C, Segan CJ, Brown N (2014) A systematic review of barriers and facilitators to participation in randomized controlled trials by indigenous people from New Zealand, Australia, Canada and the United States. Glob Health Promot [Epub ahead of print]
Lara PN, Higdon R, Lim N, Kwan K, Tanaka M, Lau D, Wun T, Welborn J, Meyers FJ, Christensen S, O'Donnell R, Richman C, Scudder SA, Tuscano J, Gandara DR, Lam KS (2001) Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. JCO 19:1728–1733
Stark N, Paskett E, Bell R, Cooper MR, Walker E, Wilson A, Tatum C (2002) Increasing participation of minorities in cancer clinical trials: summary of the “Moving Beyond the Barriers” Conference in North Carolina. J Natl Med Assoc 94:31–39
Tejada HA, Green SB, Trimble EL et al (1996) Representation of African-Americans, Hispanics, and whites in National Cancer Institute treatment trials. J Natl Cancer Inst 88:812–816
Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, McCabe M, Kaplan R, Smith M, Ungerleider R, Christian MC (2002) How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. JCO 20:2109–2117
National Institute for Health Research (2014) Clinical Research Network: About cancer research. http://www.crn.nihr.ac.uk/cancer/about-cancer-research/. Accessed 09/20 2014
Canadian Cancer Research Alliance (2011) Report on the state of cancer clinical trials in Canada. CCRA, Toronto
Cheng SK, Dietrich MS, Dilts DM (2011) Predicting accrual achievement: monitoring accrual milestones of NCI-CTEP-sponsored clinical trials. Clin Cancer Res 17:1947–1955
Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, Ellis P, Wright JR (2006) Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol 7:141–148
Biedrzycki BA (2010) Decision making for cancer clinical trial participation: a systematic review. Oncol Nurs Forum 37:E387–E399
Cox K, McGarry J (2003) Why patients don’t take part in cancer clinical trials: an overview of the literature. Eur J Cancer Care 12:114–122
Townsley CA, Selby R, Siu LL (2005) Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 23:3112–3124
Schmotzer GL (2012) Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis 22:226–230
Dixon-Woods M, Bonas S, Booth A, Jones DR, Miller T, Sutton AJ, Shaw RL, Smith JA, Young B (2006) How can systematic reviews incorporate qualitative research? A critical perspective. Qual Res 6:27–44
Beauchamp TL, Childress JF (2012) Principles of biomedical ethics. Oxford University Press, New York
Sherwin S (1998) A relational approach to autonomy in health care. In: Sherwin S, the Feminist Health Care Ethics Research Network (eds) The politics of women’s health: exploring agency and autonomy. Temple University Press, Philadelphia, pp 19–47
Paskett ED, Cooper MR, Stark N, Ricketts TC, Tropman S, Hatzell T, Aldrich T, Atkins J (2002) Clinical trial enrollment of rural patients with cancer. Cancer Pract 10:28–35
Sharkey K, Savulescu J, Aranda S, Schofield P (2010) Clinician gate-keeping in clinical research is not ethically defensible: an analysis. J Med Ethics 36:363–366
Whittemore R, Knafl K (2005) The integrative review: updated methodology. J Adv Nurs 52:546–553
Klabunde C, Kaluzny A, Ford L (1995) Community Clinical Oncology Program participation in the breast cancer prevention trial: factors affecting accrual. Cancer Epidemiol Biomarkers Prev 4:783–789
Klabunde CN, Kaluzny AD (1995) Accrual to the breast cancer prevention trial by participating Community Clinical Oncology Programs: a panel data analysis. Breast Cancer Res Treat 35:43–50
Advani AS, Atkeson B, Brown CL, Peterson BL, Fish L, Johnson JL, Gockerman JP, Gautier M (2003) Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer 97:1499–1506
Ellis PM, Butow PN, Tattersall MHN, Dunn SM, Houssami N (2001) Randomized clinical trials in oncology: understanding and attitudes predict willingness to participate. JCO 19:3554–3561
Go RS, Frisby KA, Lee JA (2006) Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer 106:426–433
Gross CP, Filardo G, Mayne ST, Krumholz HM (2005) The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer 103:483–491
Agrawal M, Grady C, Fairclough DL, Meropol NJ, Maynard K, Emanuel EJ (2006) Patients’ decision-making process regarding participation in phase I oncology research. J Clin Oncol 24:4479–4484
Avis NE, Smith KW, Link CL, Hortobagyi GN, Rivera E (2006) Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol 24:1860–1867
Catania C, De Pas T, Goldhirsch A, Radice D, Adamoli L, Medici M, Verri E, Marenghi C, de Braud F, Nole F (2008) Participation in clinical trials as viewed by the patient: understanding cultural and emotional aspects which influence choice. Oncology 74:177–187
Catt S, Langridge C, Fallowfield L, Talbot DC, Jenkins V (2011) Reasons given by patients for participating, or not, in phase 1 cancer trials. Eur J Cancer 47:1490–1497
Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME (2007) Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int 101:982–987
Jenkins V, Fallowfield L (2000) Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer 82:1783–1788
Jones JM, Nyhof-Young J, Moric J, Friedman A, Wells W, Catton P (2006) Identifying motivations and barriers to patient participation in clinical trials. J Cancer Educ 21:237–242
Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, Bartlett N, Fleming G, Cohen HJ (2003) Barriers to clinical trial participation by older women with breast cancer. JCO 21:2268–2275
Schutta KM, Burnett CB (2000) Factors that influence a patient’s decision to participate in a phase I cancer clinical trial. Oncol Nurs Forum 27:1435–1438
Truong TH, Weeks JC, Cook EF, Joffe S (2011) Altruism among participants in cancer clinical trials. Clin Trials 8:616–623
Wang LH, Tsai YF, Chen JS, Tsay PK (2011) Intention, needs, and expectations of cancer patients participating in clinical trials. Cancer Nurs 34:117–123
Wright JR, Whelan TJ, Schiff S, Dubois S, Crooks D, Haines PT, DeRosa D, Roberts RS, Gafni A, Pritchard K, Levine MN (2004) Why cancer patients enter randomized clinical trials: exploring the factors that influence their decision. JCO 22:4312–4318
Mancini J, Genève J, Dalenc F, Genre D, Monnier A, Kerbrat P, Largillier R, Serin D, Rios M, Roché H, Jimenez M, Tarpin C, the Patients’ Committee for Clinical trials of the Ligue Nationale, Julian Reynier C (2007) Decision-making and breast cancer clinical trials: how experience challenges attitudes. Contemp Clin Trials 28:684–694
Meropol NJ, Buzaglo JS, Millard J, Damjanov N, Miller SM, Ridgway C, Ross EA, Sprandio JD, Watts P (2007) Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Canc Netw 5:655–664
Sabesan S, Burgher B, Buettner P, Piliouras P, Otty Z, Varma S, Thaker D (2011) Attitudes, knowledge and barriers to participation in cancer clinical trials among rural and remote patients. Asia Pac J Clin Oncol 7:27–33
Solomon MJ, Pager CK, Young JM, Roberts R, Butow P (2003) Patient entry into randomized controlled trials of colorectal cancer treatment: factors influencing participation. Surgery 133:608–613
Weckstein DJ, Thomas CA, Emery IF, Shea BF, Fleury A, White ME, Chase E, Robinson C, Frazier S, Pilar C (2011) Assessment of perceived cost to the patient and other barriers to clinical trial participation. J Oncol Pract 7:330–333
Li J, Yu C, Jiang Y (2010) Participation in cancer clinical trials as viewed by chinese patients and their families. Oncology 79:343–348
Roberson NL (1994) Clinical trial participation. Viewpoints from racial/ethnic groups. Cancer 74:2687–2691
Llewellyn-Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C (1991) Patients’ willingness to enter clinical trials: measuring the association with perceived benefits and preference for decision participation. Soc Sci and Med 32:35–42
Melisko ME, Hassin F, Metzroth L, Moore DH, Brown B, Patel K, Rugo HS, Tripathy D (2005) Patient and physician attitudes toward breast cancer clinical trials: developing interventions based on understanding barriers. Clin Breast Cancer 6:45–54
Kim JW, Kim S, Chung Y, Kwon J, Lee H, Chung Y, Kim YJ, Oh D, Lee S, Kim D, Im S, Kim T, Heo DS, Bang Y (2008) Cancer patients’ awareness of clinical trials, perceptions on the benefit and willingness to participate: Korean perspectives. Br J Cancer 99:1593–1599
Brown DR, Topcu M (2003) Willingness to participate in clinical treatment research among older African Americans and Whites. Gerontologist 43:62–72
Kornblith AB, Kemeny MM, Peterson BL, Wheeler J, Crawford J, Bartlett N, Fleming G, Graziano S, Muss H, Cohen JH, Cancer and Leukemia Group B (2002) Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer 95:989–996
Nurgat ZA, Craig W, Campbell NC, Bissett JD, Cassidy J, Nicolson MC (2005) Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br J Cancer 92:1001–1005
LaVallie DL, Wolf FM, Jacobsen C, Buchwald D (2008) Barriers to cancer clinical trial participation among Native elders. Ethn Dis 18:210–217
Ling J, Rees E, Hardy J (2000) What influences participation in clinical trials in palliative care in a cancer centre? Eur J Cancer 36:621–626
Flessig A, Jenkins V, Fallowfield L (2001) Results of an intervention study to improve communication about randomised clinical trials of cancer therapy. Eur J Cancer 37:322–331
Cox K, Avis M (1996) Psychosocial aspects of participation in early anticancer drug trials. Report of a pilot study. Cancer Nurs 19:177–186
Coyne CA, Demian-Popescu C, Brown P (2004) Rural cancer patients’ perspectives on clinical trials: a qualitative study. J Cancer Educ 19:165–169
Ellis PM, Butow PN (1998) Focus group interviews examining attitudes to randomised trials among breast cancer patients and the general community. Aust N Z J Public Health 22:528–531
Eng M, Taylor L, Verhoef M, Ernst S, Donnelly B (2005) Understanding participation in a trial comparing cryotherapy and radiation treatment. Can J Urol 12:2607–2613
Grunfeld E, Zitzelberger L, Coristine M, Aspelund F (2002) Barriers and facilitators to enrollment in cancer clinical trials: qualitative study of the perspectives of clinical research associates. Cancer 95:1577–1583
Huizinga GA, Sleijfer DT, van de Wiel HB, van der Graaf WT (1999) Decision-making process in patients before entering phase III cancer clinical trials: a pilot study. Cancer Nurs 22:119–125
Kohara I, Inoue T (2010) Searching for a way to live to the end: decision-making process in patients considering participation in cancer phase I clinical trials. Oncol Nurs Forum 37:E124–32
Kvale EA, Woodby L, Williams BR (2010) The experience of older patients with cancer in phase 1 clinical trials: a qualitative case series. Am J Hosp Palliat Care 27:474–481
Madsen SM, Holm S, Riis P (2007) Attitudes towards clinical research among cancer trial participants and non-participants: an interview study using a grounded theory approach. J Med Ethics 33:234–240
Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA (2011) Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 64:1127–1136
Schaefer KM, Ladd E, Gergits MA, Gyauch L (2001) Backing and forthing: the process of decision making by women considering participation in a breast cancer prevention trial. Oncol Nurs Forum 28:703–709
Shah A, Efstathiou JA, Paly JJ, Halpern SD, Bruner DW, Christodouleas JP, Coen JJ, Deville C Jr, Vapiwala N, Shipley WU, Zietman AL, Hahn SM, Bekelman JE (2012) Prospective preference assessment of patients’ willingness to participate in a randomized controlled trial of intensity-modulated radiotherapy versus proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 83:e13–9
Shannon-Dorcy K, Drevdahl DJ (2011) “I had already made up my mind”: patients and caregivers’ perspectives on making the decision to participate in research at a US cancer referral center. Cancer Nurs 34:428–433
Stevens T, Ahmedzai SH (2004) Why do breast cancer patients decline entry into randomised trials and how do they feel about their decision later: a prospective, longitudinal, in-depth interview study. Patient Educ Couns 52:341–348
Ellis PM, Butow P (1998) Focus group interviews examining attitudes to randomized trials among breast cancer patients and the general community. Aust N Z J Public Health 22:528–531
Madsen SM, Mirza MR, Holm S, Hilsted KL, Kampmann K, Riis P (2002) Attitudes towards clinical research amongst participants and nonparticipants. J Intern Med 251:156
Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada. (2010) Tri-Council Policy Statement: ethical conduct for research involving humans
Donchin A (2001) Understanding autonomy relationally: toward a reconfiguration of bioethical principles. J Med Philos 26:365–386
Ho A (2008) Relational autonomy or undue pressure? Family’s role in medical decision‐making. Scand J Caring Sci 22:128–135
Mackenzie C, Stoljar N (2000) Introduction: autonomy refigured. In: anonymous relational autonomy: feminist perspectives on autonomy, agency, and the social self. Oxford University Press, New York, pp 35–51
Robinson CA (2011) Advance care planning: re-visioning our ethical approach. Can J Nurs Res 43:18–37
Gilbar R (2007) Communicating genetic information in the family: the familial relationship as the forgotten factor. J Med Ethics 33:390–393
Candib LM (2002) Truth telling and advance planning at the end of life: problems with autonomy in a multicultural world. Fam Syst Health 20:213–228
Eliott J, Olver I (2008) Choosing between life and death: patient and family perceptions of the decision not to resuscitate the terminally ill cancer patient. Bioethics 22:179–189
Freedman B (1987) Equipoise and the ethics of clinical research. N Engl J Med 317:141–145
Acknowledgments
Funding for this project was received from the Canadian Institutes of Health Research (CIHR)–Institute Ethics office. JAHB was supported by a CIHR Doctoral Research Award and a CIHR Psychosocial Oncology Research Training (PORT) fellowship. LGB was supported by a CIHR New Investigator Award (2008-2013).
Conflict of interest
The authors (JAHB and LGB) declare that they have no competing interests.
Authors’ contributions
JAHB carried out this research as part of her doctoral dissertation. She collected and analyzed the data and drafted the manuscript. LGB participated in the presentation of ideas in the manuscript and reviewed and provided significant feedback on the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendices
Rights and permissions
About this article
Cite this article
Bell, J.A.H., Balneaves, L.G. Cancer patient decision making related to clinical trial participation: an integrative review with implications for patients’ relational autonomy. Support Care Cancer 23, 1169–1196 (2015). https://doi.org/10.1007/s00520-014-2581-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2581-9