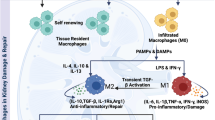
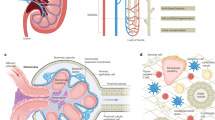
Abstract
The kidneys are crucial for filtering blood, managing overall body water, electrolyte, and acid–base balance, and regulating blood pressure. They remove metabolic waste products, toxins, and drugs. In addition, they limit inflammation by clearing cytokines and reduce immune cell activation by removing bacterial components. Dendritic cells (DCs) in the kidney maintain peripheral tolerance. About 85% of filtered water is reabsorbed by the proximal tubule, exposing distal nephron cells to high concentrations of low molecular weight antigens. These antigens are captured by DCs, helping to inactivate potentially autoreactive T cells and maintain tolerance to circulating antigens. In kidney failure, immune function is severely compromised due to the retention of toxins and cytokines, which activate immune cells and increase systemic inflammation. The kidneys are also vulnerable to immune-mediated diseases. Loss of immune homeostasis, characterized by over- or under-activity of the immune response, can adversely affect kidney function. With advances in immunology and cellular biology, biologic therapies targeting various pathways involved in the pathophysiology of kidney diseases are being developed. In this review, the immunologic aspects of kidney diseases and focus on cytokine-based therapies that may hold promise for the treatment of kidney diseases in the future will be presented.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
References
Kurts C, Panzer U, Anders HJ, Rees AJ (2013) The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol 13:738–753
Betjes MG (2013) Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol 9:255–265
Tecklenborg J, Clayton D, Siebert S, Coley SM (2018) The role of the immune system in kidney disease. Clin Exp Immunol 192:142–150
Eleftheriadis T, Crespo M (2022) Molecular aspects of renal immunology: current status and future perspectives. Int J Mol Sci 23:4040
Hutton HL, Ooi JD, Holdsworth SR, Kitching AR (2016) The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton) 21:736–744
Joosten LA, Netea MG, Dinarello CA (2013) Interleukin-1beta in innate inflammation, autophagy and immunity. Semin Immunol 25:41–424
Ferrari D, Pizzirani C, Adinolfi E et al (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176:387–3883
Kayagaki N, Warming S, Lamkanfi M et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121
Gabay C, Lamacchia C, Palmer G (2010) IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 6:232–241
Novick D, Kim S, Kaplanski G, Dinarello CA (2013) Interleukin-18, more than a Th1 cytokine. Semin Immunol 25:439–448
Kitching AR, Hickey MJ (2022) Immune cell behaviour and dynamics in the kidney - insights from in vivo imaging. Nat Rev Nephrol 18:22–37
Sepe V, Libetta C, Gregorini M, Rampino T (2022) The innate immune system in human kidney inflammaging. J Nephrol 35:381–395
Chan EY, Yap DY, Colucci M, Ma AL, Parekh RS, Tullus K (2023) Use of rituximab in childhood idiopathic nephrotic syndrome. Clin J Am Soc Nephrol 18:533–548
Provenzano M, Hu L, Tringali E et al (2024) Improving kidney disease care: one giant leap for nephrology. Biomedicines 12:828
Leibler C, Thiolat A, Elsner RA, El Karoui K, Samson C, Grimbert P (2019) Costimulatory blockade molecules and B-cell-mediated immune response: current knowledge and perspectives. Kidney Int 95:774–786
Antonucci L, Thurman JM, Vivarelli M (2024) Complement inhibitors in pediatric kidney diseases: new therapeutic opportunities. Pediatr Nephrol 39:1387–1404
Kwun J, Manook M, Page E, Burghuber C, Hong J, Knechtle SJ (2017) Crosstalk between T and B cells in the germinal center after transplantation. Transplantation 101:704–712
Karras A, Jayne D (2014) New biologics for glomerular disease on the horizon. Nephron Clin Pract 128:283–291
Lucherini OM, Rigante D, Sota J et al (2018) Updated overview of molecular pathways involved in the most common monogenic autoinflammatory diseases. Clin Exp Rheumatol 36:3–9
Hegazy MT, Fayed A, Nuzzolese R, Sota J, Ragab G (2023) Autoinflammatory diseases and the kidney. Immunol Res 71:578–587
Lorenz G, Darisipudi MN, Anders H-J (2013) Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant 29:41–48
Zhao J, Rui HL, Yang M, Sun LJ, Dong HR, Cheng H (2019) CD36-mediated lipid accumulation and activation of NLRP3 inflammasome lead to podocyte injury in obesity-related glomerulopathy. Mediators Inflamm 2019:3172647
Tsai YL, Hua KF, Chen A et al (2017) NLRP3 inflammasome: pathogenic role and potential therapeutic target for IgA nephropathy. Sci Rep 7:41123
Martin-Higueras C, Ludwig-Portugall I, Hoppe B, Kurts C (2019) Targeting kidney inflammation as a new therapy for primary hyperoxaluria? Nephrol Dial Transplant 34:908–914
Anders HJ, Suarez-Alvarez B, Grigorescu M et al (2018) The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int 93:656–669
Huang G, Bao J, Shao X, Zhou W, Wu B, Ni Z, Wang L (2020) Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci 254:117791
Karam S, Haidous M, Royal V, Leung N (2023) Renal AA amyloidosis: presentation, diagnosis, and current therapeutic options: a review. Kidney Int 103:473–484
Gottenberg JE, Merle-Vincent F, Bentaberry F et al (2003) Anti-tumor necrosis factor alpha therapy in fifteen patients with AA amyloidosis secondary to inflammatory arthritides: a follow-up report of tolerability and efficacy. Arthritis Rheum 48:2019–2024
Zhang J, Rudemiller NP, Patel MB et al (2016) Interleukin-1 Receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 Co-transporter in the nephron. Cell Metab 23:360–368
Allison SJ (2016) Hypertension: IL-1 receptor-induced sodium reabsorption in hypertension. Nat Rev Nephrol 12:126
Mulay SR, Kulkarni OP, Rupanagudi KV et al (2013) Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J Clin Invest 123:236–246
Dember LM, Hung A, Mehrotra R et al (2022) A randomized controlled pilot trial of anakinra for hemodialysis inflammation. Kidney Int 102:1178–1187
Ridker PM, MacFadyen JG, Glynn RJ et al (2018) Inhibition of Interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 71:2405–2414
Mulders-Manders CM, Baas MC, Molenaar FM, Simon A (2017) Peri- and postoperative treatment with the interleukin-1 receptor antagonist anakinra is safe in patients undergoing renal transplantation: case series and review of the literature. Front Pharmacol 8:342
Yazısız V, Yılmaz VT, Uçar İ et al (2021) The use of anti-interleukin-1 agents and tumor necrosis factor-alpha inhibitors in renal transplant recipients. Arch Rheumatol 36:366–374
Daemen MA, Denecker G, van’t Veer C, Wolfs TG, Vandenabeele P, Buurman WA (2001) Activated caspase-1 is not a central mediator of inflammation in the course of ischemia-reperfusion. Transplantation 71:778-784
Chandran S, Tang Q (2022) Impact of interleukin-6 on T cells in kidney transplant recipients. Am J Transplant Suppl 4:18–27
Doberer K, Duerr M, Halloran PF et al (2021) A randomized clinical trial of anti-IL-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol 32:708–722
Chandran S, Leung J, Hu C, Laszik ZG, Tang Q, Vincenti FG (2021) Interleukin-6 blockade with tocilizumab increases Tregs and reduces T effector cytokines in renal graft inflammation: a randomized controlled trial. Am J Transplant 21:2543–2554
Zhao Y, Cooper DKC, Wang H et al (2019) Potential pathological role of pro-inflammatory cytokines (IL-6, TNF-α, and IL-17) in xenotransplantation. Xenotransplantation 26:e12502
Pergola PE, Devalaraja M, Fishbane S et al (2021) Ziltivekimab for treatment of anemia of inflammation in patients on hemodialysis: results from a phase 1/2 multicenter, randomized, double-blind, placebo-controlled trial. J Am Soc Nephrol 32:211–222
Pergola PE, Davidson M, Jensen C et al (2024) Effect of ziltivekimab on determinants of hemoglobin in patients with CKD stage 3–5: an analysis of a randomized trial (RESCUE). J Am Soc Nephrol 35:74–84
Almenara-Tejederas M, Alonso-García F, Aguillera-Morales WA, de la Prada-Álvares F, Salgueira-Lazo M (2022) Blockade of interleukin-6 as a possible therapeutic target for AA amyloidosis. Nefrologia (Engl Ed) 42:28–32
Wynne BM, Samson TK, Moyer HC et al (2022) Interleukin 6 mediated activation of the mineralocorticoid receptor in the aldosterone-sensitive distal nephron. Am J Physiol Cell Physiol 323:C1512–C1523
Huang MY, Chaturvedi LS, Koul S, Koul HK (2005) Oxalate stimulates IL-6 production in HK-2 cells, a line of human renal proximal tubular epithelial cells. Kidney Int 68:497–503
Shee K, Stoller ML (2022) Perspectives in primary hyperoxaluria - historical, current and future clinical interventions. Nat Rev Urol 19:137–146
Gaffen SL (2009) Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9:556–567
Ramani K, Biswas PS (2016) Emerging roles of the Th17/IL-17-axis in glomerulonephritis. Cytokine 77:238–244
Apostolidis SA, Crispín JC, Tsokos GC (2011) IL-17-producing T cells in lupus nephritis. Lupus 20:120–124
Haouami Y, Dhaouadi T, Sfar I et al (2018) The role of IL-23/IL-17 axis in human kidney allograft rejection. J Leukoc Biol 104:1229–1239
Wen Q, Hang G, Wang Y, Yu Z, Wang H, Chen B (2023) Changes and significance of interleukin 17 expression in patients after renal transplantation. Transplant Proc 55:562–568
Rodrigues-Diez RR, Tejera-Muñoz A, Orejudo M et al (2021) Interleukin-17A: potential mediator and therapeutic target in hypertension. Nefrologia (Engl Ed) 41:244–257
Zhang F, Yin J, Liu L et al (2023) IL-17C neutralization protects the kidney against acute injury and chronic injury. EBioMedicine 92:104607
Kronbichler A, Leierer J, Oh J, Meijers B, Shin JI (2016) Immunologic changes implicated in the pathogenesis of focal segmental glomerulosclerosis. Biomed Res Int 2016:2150451
Joy MS, Gipson DS, Powell L et al (2010) Phase 1 trial of adalimumab in focal segmental glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am J Kidney Dis 55:50–60
Trachtman H, Fervenza FC, Gipson DS et al (2011) A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79:1236–1243
Vincenti F, Fervenza FC, Campbell KN et al (2017) Focal Segmental Glomerulosclerosis Study Group. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep 2:800–810
Kroschinsky F, Stölzel F, von Bonin S et al (2017) Intensive Care in Hematological and Oncological Patients (iCHOP) Collaborative Group. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care 21:89
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ (2010) The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9:325–338
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leventoğlu, E., Bakkaloğlu, S.A. A new era in the treatment of kidney diseases: NLRP3 inflammasome and cytokine-targeted therapies. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06578-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00467-024-06578-0