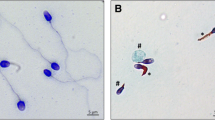
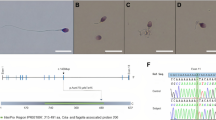
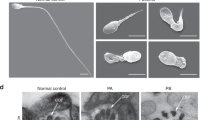
Abstract
Spermatozoa contain highly specialized structural features reflecting unique functions required for fertilization. Among them, the flagellum is a sperm-specific organelle required to generate the motility, which is essential to reach the egg. The flagellum integrity is, therefore, critical for normal sperm function and flagellum defects consistently lead to male infertility due to reduced or absent sperm motility defined as asthenozoospermia. Multiple morphological abnormalities of the flagella (MMAF), also called short tails, is among the most severe forms of sperm flagellum defects responsible for male infertility and is characterized by the presence in the ejaculate of spermatozoa being short, coiled, absent and of irregular caliber. Recent studies have demonstrated that MMAF is genetically heterogeneous which is consistent with the large number of proteins (over one thousand) localized in the human sperm flagella. In the past 5 years, genomic investigation of the MMAF phenotype allowed the identification of 18 genes whose mutations induce MMAF and infertility. Here we will review information about those genes including their expression pattern, the features of the encoded proteins together with their localization within the different flagellar protein complexes (axonemal or peri-axonemal) and their potential functions. We will categorize the identified MMAF genes following the protein complexes, functions or biological processes they may be associated with, based on the current knowledge in the field.




Similar content being viewed by others
References
Alexandre C, Bisson JP, David G (1978) Total asthenospermia with an ultrastructural anomaly of the flagellum in two sterile brothers (author's transl). J Gynecol Obstet Biol Reprod (Paris) 7:31–38
Amaral A, Castillo J, Estanyol JM, Ballesca JL, Ramalho-Santos J, Oliva R (2013) Human sperm tail proteome suggests new endogenous metabolic pathways. Mol Cell Proteomics 12:330–342. https://doi.org/10.1074/mcp.M112.020552
Amiri-Yekta A et al (2016) Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations. Hum Reprod 31:2872–2880. https://doi.org/10.1093/humrep/dew262
Auger J, Jouannet P, Eustache F (2016) Another look at human sperm morphology. Hum Reprod 31:10–23. https://doi.org/10.1093/humrep/dev251
Auguste Y et al (2018) Loss of calmodulin- and radial-spoke-associated complex protein CFAP251 leads to immotile spermatozoa lacking mitochondria and infertility in men. Am J Hum Genet 103:413–420. https://doi.org/10.1016/j.ajhg.2018.07.013
Baccetti B, Collodel G, Estenoz M, Manca D, Moretti E, Piomboni P (2005) Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum Reprod 20:2790–2794
Barthelemy C, Tharanne MJ, Lebos C, Lecomte P, Lansac J (1990) Tail stump spermatozoa: morphogenesis of the defect. An ultrastructural study of sperm and testicular biopsy. Andrologia 22:417–425
Ben Khelifa M et al (2014) Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet 94:95–104. https://doi.org/10.1016/j.ajhg.2013.11.017
Beneke T et al (2019) Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog 15:e1007828. https://doi.org/10.1371/journal.ppat.1007828
Bettencourt-Dias M, Hildebrandt F, Pellman D, Woods G, Godinho SA (2011) Centrosomes and cilia in human disease. Trends Genet 27:307–315. https://doi.org/10.1016/j.tig.2011.05.004
Beurois J et al (2019) CFAP70 mutations lead to male infertility due to severe astheno-teratozoospermia. A case report. Hum Reprod Oxf Engl 34:2071–2079. https://doi.org/10.1093/humrep/dez166
Blue E et al (2018) Variation in cilia protein genes and progression of lung disease in cystic fibrosis. Ann Am Thorac Soc 15:440–448. https://doi.org/10.1513/AnnalsATS.201706-451OC
Brown PR, Miki K, Harper DB, Eddy EM (2003) A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol Reprod 68:2241–2248. https://doi.org/10.1095/biolreprod.102.013466
Cao W, Haig-Ladewig L, Gerton GL, Moss SB (2006) Adenylate kinases 1 and 2 are part of the accessory structures in the mouse sperm flagellum. Biol Reprod 75:492–500. https://doi.org/10.1095/biolreprod.106.053512
Carrera A, Gerton GL, Moss SB (1994) The major fibrous sheath polypeptide of mouse sperm: structural and functional similarities to the A-kinase anchoring proteins. Dev Biol 165:272–284. https://doi.org/10.1006/dbio.1994.1252
Chemes H (2012) Sperm centrioles and their dual role in flagellogenesis and cell cycle of the zygote, pp 33–48. https://doi.org/10.1007/978-1-62703-035-9_2
Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC (1987) Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril 48:664–669
Chung MI et al (2014) Coordinated genomic control of ciliogenesis and cell movement by RFX2. eLife 3:e01439. https://doi.org/10.7554/eLife.01439
Coates JC (2003) Armadillo repeat proteins: beyond the animal kingdom. Trends Cell Biol 13:463–471
Cooper TG et al (2010) World Health Organization reference values for human semen characteristics. Hum Reprod Update 16:231–245. https://doi.org/10.1093/humupd/dmp048
Coutton C et al (2015) Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update 21:455–485. https://doi.org/10.1093/humupd/dmv020
Coutton C et al (2019) Bi-allelic mutations in ARMC2 Lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am J Hum Genet 104:331–340. https://doi.org/10.1016/j.ajhg.2018.12.013
Coutton C et al (2018) Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat Commun 9:686. https://doi.org/10.1038/s41467-017-02792-7
Curi SM et al (2003) Asthenozoospermia: analysis of a large population. Arch Androl 49:343–349
Darde TA et al (2019) The ReproGenomics Viewer: a multi-omics and cross-species resource compatible with single-cell studies for the reproductive science community. Bioinformatics. https://doi.org/10.1093/bioinformatics/btz047
Darde TA et al (2015) The ReproGenomics Viewer: an integrative cross-species toolbox for the reproductive science community. Nucleic Acids Res 43:W109–W116. https://doi.org/10.1093/nar/gkv345
Dong FN et al (2018) Absence of CFAP69 causes male infertility due to multiple morphological abnormalities of the flagella in human and mouse. Am J Hum Genet 102:636–648. https://doi.org/10.1016/j.ajhg.2018.03.007
Dong L et al (2016) The role of ubiquitin-proteasome pathway in spermatogenesis. Yi Chuan Hered 38:791–800. https://doi.org/10.16288/j.yczz.16-120
Dymek EE, Heuser T, Nicastro D, Smith EF (2011) The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol Biol Cell 22:2520–2531. https://doi.org/10.1091/mbc.E11-03-0271
Dymek EE, Smith EF (2007) A conserved CaM- and radial spoke associated complex mediates regulation of flagellar dynein activity. J Cell Biol 179:515–526. https://doi.org/10.1083/jcb.200703107
Dzeja P, Terzic A (2009) Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci 10:1729–1772. https://doi.org/10.3390/ijms10041729
Eddy EM (2007) The scaffold role of the fibrous sheath. Soc Reprod Fertil Suppl 65:45–62
Eddy EM, Toshimori K, O'Brien DA (2003) Fibrous sheath of mammalian spermatozoa. Microsc Res Tech 61:103–115. https://doi.org/10.1002/jemt.10320
Escalier D (2006) Arrest of flagellum morphogenesis with fibrous sheath immaturity of human spermatozoa. Andrologia 38:54–60
Escalier D, Albert M (2006) New fibrous sheath anomaly in spermatozoa of men with consanguinity. Fertil Steril 86(219):e211–219
Escalier D, Toure A (2012) Morphological defects of sperm flagellum implicated in human male infertility. Med Sci 28:503–511. https://doi.org/10.1051/medsci/2012285015
Fauque P et al (2009) From ultrastructural flagellar sperm defects to the health of babies conceived by ICSI. Reprod Biomed Online 19:326–336
Fernandez-Gonzalez A, Kourembanas S, Wyatt TA, Mitsialis SA (2009) Mutation of murine adenylate kinase 7 underlies a primary ciliary dyskinesia phenotype. Am J Respir Cell Mol Biol 40:305–313. https://doi.org/10.1165/rcmb.2008-0102OC
Fu G et al (2018) The I1 dynein-associated tether and tether head complex is a conserved regulator of ciliary motility. Mol Biol Cell 29:1048–1059. https://doi.org/10.1091/mbc.E18-02-0142
Gershoni M et al (2017) A familial study of azoospermic men identifies three novel causative mutations in three new human azoospermia genes. Genet Med Off J Am Coll Med Genet 19:998–1006. https://doi.org/10.1038/gim.2016.225
He X et al (2019) Novel homozygous CFAP69 mutations in humans and mice cause severe asthenoteratospermia with multiple morphological abnormalities of the sperm flagella. J Med Genet 56:96–103. https://doi.org/10.1136/jmedgenet-2018-105486
Heuser T, Barber CF, Lin J, Krell J, Rebesco M, Porter ME, Nicastro D (2012a) Cryoelectron tomography reveals doublet-specific structures and unique interactions in the I1 dynein. Proc Natl Acad Sci USA 109:E2067–2076. https://doi.org/10.1073/pnas.1120690109
Heuser T, Dymek EE, Lin J, Smith EF, Nicastro D (2012b) The CSC connects three major axonemal complexes involved in dynein regulation. Mol Biol Cell 23:3143–3155. https://doi.org/10.1091/mbc.E12-05-0357
Hiraki M, Nakazawa Y, Kamiya R, Hirono M (2007) Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr Biol 17:1778–1783. https://doi.org/10.1016/j.cub.2007.09.021
Hom EF et al (2011) A unified taxonomy for ciliary dyneins. Cytoskeleton 68:555–565. https://doi.org/10.1002/cm.20533
Horani A, Ferkol TW, Dutcher SK, Brody SL (2016) Genetics and biology of primary ciliary dyskinesia. Paediatr Respir Rev 18:18–24. https://doi.org/10.1016/j.prrv.2015.09.001
Hu J et al (2019) ENU-induced mutant allele of Dnah1, ferf1, causes abnormal sperm behavior and fertilization failure in mice. Mol Reprod Dev 86:416–425. https://doi.org/10.1002/mrd.23120
Hussain MS et al (2012) A truncating mutation of CEP135 causes primary microcephaly and disturbed centrosomal function. Am J Hum Genet 90:871–878. https://doi.org/10.1016/j.ajhg.2012.03.016
Imtiaz F, Allam R, Ramzan K, Al-Sayed M (2015) Variation in DNAH1 may contribute to primary ciliary dyskinesia. BMC Med Genet 16:14. https://doi.org/10.1186/s12881-015-0162-5
Inaba K (2007) Molecular basis of sperm flagellar axonemes: structural and evolutionary aspects. Ann N Y Acad Sci 1101:506–526. https://doi.org/10.1196/annals.1389.017
Inaba K (2011) Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod 17:524–538. https://doi.org/10.1093/molehr/gar034
Ishikawa H, Marshall WF (2011) Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol 12:222–234. https://doi.org/10.1038/nrm3085
Kherraf ZE et al (2018) A homozygous ancestral SVA-insertion-mediated deletion in WDR66 induces multiple morphological abnormalities of the sperm flagellum and male infertility. Am J Hum Genet 103:400–412. https://doi.org/10.1016/j.ajhg.2018.07.014
Kherraf ZE et al (2019) Whole exome sequencing of men with multiple morphological abnormalities of the sperm flagella reveals novel homozygous QRICH2 mutations. Clin Genet 5:5. https://doi.org/10.1111/cge.13604
Kierszenbaum AL (2002) Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev 63:1–4. https://doi.org/10.1002/mrd.10179
King SM (2012) Integrated control of axonemal dynein AAA(+) motors. J Struct Biol 179:222–228. https://doi.org/10.1016/j.jsb.2012.02.013
Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA (2007) Plk4-induced centriole biogenesis in human cells. Dev Cell 13:190–202. https://doi.org/10.1016/j.devcel.2007.07.002
Knowles MR, Zariwala M, Leigh M (2016) Primary ciliary dyskinesia. Clin Chest Med 37:449–461. https://doi.org/10.1016/j.ccm.2016.04.008
Kobayashi D, Takeda H (2012) Ciliary motility: the components and cytoplasmic preassembly mechanisms of the axonemal dyneins. Differentiation 83:S23–29. https://doi.org/10.1016/j.diff.2011.11.009
Kraatz S et al (2016) The human centriolar protein CEP135 contains a two-stranded coiled-coil domain critical for microtubule binding. Structure 24:1358–1371. https://doi.org/10.1016/j.str.2016.06.011
Kubo T, Hou Y, Cochran DA, Witman GB, Oda T (2018) A microtubule-dynein tethering complex regulates the axonemal inner dynein f (I1). Mol Biol Cell 29:1060–1074. https://doi.org/10.1091/mbc.E17-11-0689
Kurkowiak M, Zietkiewicz E, Witt M (2015) Recent advances in primary ciliary dyskinesia genetics. J Med Genet 52:1–9. https://doi.org/10.1136/jmedgenet-2014-102755
Lehti MS, Sironen A (2017) Formation and function of sperm tail structures in association with sperm motility defects. Biol Reprod 97:522–536. https://doi.org/10.1093/biolre/iox096
Lehti MS, Zhang FP, Kotaja N, Sironen A (2017) SPEF2 functions in microtubule-mediated transport in elongating spermatids to ensure proper male germ cell differentiation. Development 144:2683–2693. https://doi.org/10.1242/dev.152108
Li Y et al (2016) DNAH6 and its interactions with PCD genes in heterotaxy and primary ciliary dyskinesia. PLoS Genet 12:e1005821. https://doi.org/10.1371/journal.pgen.1005821
Li L et al (2018) DNAH6 is a novel candidate gene associated with sperm head anomaly. Andrologia. https://doi.org/10.1111/and.12953
Li W et al (2019a) Bi-allelic mutations of CFAP251 cause sperm flagellar defects and human male infertility. J Hum Genet. https://doi.org/10.1038/s10038-018-0520-1
Li W et al (2019b) Bi-allelic mutations in CFAP65 cause male infertility with multiple morphological abnormalities of the sperm flagella in humans and mice. J Med Genet. https://doi.org/10.1136/jmedgenet-2019-106344
Li Y et al (2019c) DNAH2 is a novel candidate gene associated with multiple morphological abnormalities of the sperm flagella. Clin Genet 95:590–600. https://doi.org/10.1111/cge.13525
Linck RW, Chemes H, Albertini DF (2016) The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J Assist Reprod Genet 33:141–156. https://doi.org/10.1007/s10815-016-0652-1
Liu C et al (2019a) Bi-allelic mutations in TTC29 cause male subfertility with asthenoteratospermia in humans and mice. Am J Hum Genet. https://doi.org/10.1016/j.ajhg.2019.10.010
Liu C et al (2019b) Homozygous mutations in SPEF2 induce multiple morphological abnormalities of the sperm flagella and male infertility. J Med Genet. https://doi.org/10.1136/jmedgenet-2019-106011
Liu H et al (2017) IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation. Biol Reprod 96:993–1006. https://doi.org/10.1093/biolre/iox029
Liu W et al (2019a) Bi-allelic mutations in TTC21A induce asthenoteratospermia in humans and mice. Am J Hum Genet 104:738–748. https://doi.org/10.1016/j.ajhg.2019.02.020
Liu W et al (2019b) Loss-of-function mutations in SPEF2 cause multiple morphological abnormalities of the sperm flagella (MMAF). J Med Genet 56:678–684. https://doi.org/10.1136/jmedgenet-2018-105952
Liu W et al (2019c) Homozygous loss-of-function mutations in FSIP2 cause male infertility with asthenoteratospermia. J Genet Genomics Yi Chuan Xue Bao 46:53–56. https://doi.org/10.1016/j.jgg.2018.09.006
Lorès P et al (2018) Homozygous missense mutation L673P in adenylate kinase 7 (AK7) leads to primary male infertility and multiple morphological anomalies of the flagella but not to primary ciliary dyskinesia. Hum Mol Genet 27:1196–1211. https://doi.org/10.1093/hmg/ddy034
Lorès P et al (2019) Mutations in TTC29, encoding an evolutionarily conserved axonemal protein, result in asthenozoospermia and male infertility. Am J Hum Genet 1:1. https://doi.org/10.1016/j.ajhg.2019.10.007
Martinez G et al (2018) Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum Reprod 33:1973–1984. https://doi.org/10.1093/humrep/dey264
Meccariello R et al (2014) Molecular chaperones, cochaperones, and ubiquitination/deubiquitination system: involvement in the production of high quality spermatozoa. BioMed Res Int 2014:561426. https://doi.org/10.1155/2014/561426
Meienberg J, Bruggmann R, Oexle K, Matyas G (2016) Clinical sequencing: is WGS the better WES? Hum Genet 135:359–362. https://doi.org/10.1007/s00439-015-1631-9
Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM (2002) Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol 248:331–342
Milara J, Armengot M, Mata M, Morcillo EJ, Cortijo J (2010) Role of adenylate kinase type 7 expression on cilia motility: possible link in primary ciliary dyskinesia. Am J Rhinol Allergy 24:181–185. https://doi.org/10.2500/ajra.2010.24.3468
Morimoto Y et al (2019) Nonsense mutation in CFAP43 causes normal-pressure hydrocephalus with ciliary abnormalities. Neurology 92:e2364–e2374. https://doi.org/10.1212/WNL.0000000000007505
Mottier-Pavie V, Megraw TL (2009) Drosophila bld10 is a centriolar protein that regulates centriole, basal body, and motile cilium assembly. Mol Biol Cell 20:2605–2614. https://doi.org/10.1091/mbc.E08-11-1115
Myster SH, Knott JA, O'Toole E, Porter ME (1997) The Chlamydomonas Dhc1 gene encodes a dynein heavy chain subunit required for assembly of the I1 inner arm complex. Mol Biol Cell 8:607–620. https://doi.org/10.1091/mbc.8.4.607
Myster SH, Knott JA, Wysocki KM, O'Toole E, Porter ME (1999) Domains in the 1alpha dynein heavy chain required for inner arm assembly and flagellar motility in Chlamydomonas. J Cell Biol 146:801–818
Neesen J et al (2001) Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum Mol Genet 10:1117–1128
Nicastro D, Schwartz C, Pierson J, Gaudette R, Porter ME, McIntosh JR (2006) The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313:944–948. https://doi.org/10.1126/science.1128618
Norling A et al (2014) Identification of a duplication within the GDF9 gene and novel candidate genes for primary ovarian insufficiency (POI) by a customized high-resolution array comparative genomic hybridization platform. Hum Reprod Oxf Engl 29:1818–1827. https://doi.org/10.1093/humrep/deu149
Nsota Mbango J-F, Coutton C, Arnoult C, Ray P, Toure A (2019) Genetic causes of male infertility: snapshot on morphological abnormalities of the sperm flagellum. Basic Clin Androl 29:2. https://doi.org/10.1186/s12610-019-0083-9 (eCollection 2019)
Ohmori K, Matsuda T, Horii Y, Yoshida O (1993) Three cases with different types of short-tailed spermatozoa. Urol Int 50:174–178. https://doi.org/10.1159/000282478
Ohta T, Essner R, Ryu JH, Palazzo RE, Uetake Y, Kuriyama R (2002) Characterization of Cep135, a novel coiled-coil centrosomal protein involved in microtubule organization in mammalian cells. J Cell Biol 156:87–99. https://doi.org/10.1083/jcb.200108088
Pazour GJ, Agrin N, Leszyk J, Witman GB (2005) Proteomic analysis of a eukaryotic cilium. J Cell Biol 170:103–113. https://doi.org/10.1083/jcb.200504008
Peifer M, Berg S, Reynolds AB (1994) A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76:789–791. https://doi.org/10.1016/0092-8674(94)90353-0
Pigino G, Ishikawa T (2012) Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture 2:50–58
Pigino G et al (2011) Cryoelectron tomography of radial spokes in cilia and flagella. J Cell Biol 195:673–687. https://doi.org/10.1083/jcb.201106125
Ross A, Christie S, Edmond P (1973) Ultrastructural tail defects in the spermatozoa from two men attending a subfertility clinic. J Reprod Fertil 32:243–251. https://doi.org/10.1530/jrf.0.0320243
San Agustin JT, Pazour GJ, Witman GB (2015) Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol Biol Cell 26:4358–4372. https://doi.org/10.1091/mbc.E15-08-0578
Schatten H, Sun QY (2009) The role of centrosomes in mammalian fertilization and its significance for ICSI. Mol Hum Reprod 15:531–538. https://doi.org/10.1093/molehr/gap049
Sha Y et al (2019) Biallelic mutations in Sperm flagellum 2 cause human multiple morphological abnormalities of the sperm flagella (MMAF) phenotype. Clin Genet 96:385–393. https://doi.org/10.1111/cge.13602
Sha Y et al (2017) DNAH1 gene mutations and their potential association with dysplasia of the sperm fibrous sheath and infertility in the Han Chinese population. Fertil Steril 107:1312–1318. https://doi.org/10.1016/j.fertnstert.2017.04.007
Sha YW et al (2019a) Patients with multiple morphological abnormalities of the sperm flagella harbouring CFAP44 or CFAP43 mutations have a good pregnancy outcome following intracytoplasmic sperm injection. Andrologia 51:e13151. https://doi.org/10.1111/and.13151
Sha YW et al (2019b) Novel mutations in CFAP44 and CFAP43 cause multiple morphological abnormalities of the sperm flagella (MMAF). Reprod Sci Thousand Oaks Calif 26:26–34. https://doi.org/10.1177/1933719117749756
Sha YW, Xu X, Mei LB, Li P, Su ZY, He XQ, Li L (2017) A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF). Gene 633:48–53. https://doi.org/10.1016/j.gene.2017.08.033
Shamoto N, Narita K, Kubo T, Oda T, Takeda S (2018) CFAP70 is a novel axoneme-binding protein that localizes at the base of the outer dynein arm and regulates ciliary motility. Cells. https://doi.org/10.3390/cells7090124
Shen Y et al (2019) Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat Commun 10:433. https://doi.org/10.1038/s41467-018-08182-x
Sironen A, Hansen J, Thomsen B, Andersson M, Vilkki J, Toppari J, Kotaja N (2010) Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein. Biol Reprod 82:580–590. https://doi.org/10.1095/biolreprod.108.074971
Sironen A et al (2011) Loss of SPEF2 function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol Reprod 85:690–701. https://doi.org/10.1095/biolreprod.111.091132
Skerget S, Rosenow MA, Petritis K, Karr TL (2015) Sperm proteome maturation in the mouse epididymis. PLoS ONE 10:e0140650. https://doi.org/10.1371/journal.pone.0140650
Smith TF (2008) Diversity of WD-repeat proteins. Subcell Biochem 48:20–30. https://doi.org/10.1007/978-0-387-09595-0_3
Talaga AK, Dong FN, Reisert J, Zhao H (2017) Cilia- and flagella-associated protein 69 regulates olfactory transduction kinetics in mice. J Neurosci 37:5699–5710. https://doi.org/10.1523/JNEUROSCI.0392-17.2017
Tang S et al (2017) Bi-allelic mutations in CFAP43 and CFAP44 Cause male infertility with multiple morphological abnormalities of the sperm flagella. Am J Hum Genet 100:854–864. https://doi.org/10.1016/j.ajhg.2017.04.012
Tarnasky H, Cheng M, Ou Y, Thundathil JC, Oko R, van der Hoorn FA (2010) Gene trap mutation of murine outer dense fiber protein-2 gene can result in sperm tail abnormalities in mice with high percentage chimaerism. BMC Dev Biol 10:67. https://doi.org/10.1186/1471-213X-10-67
Terquem A, Dadoune JP (1980) Structural anomalies in disorders human of sperm motility in 60 cases of asthenospermia. Bull Assoc Anat (Nancy) 64:567–576
Toure A, Rode B, Hunnicutt GR, Escalier D, Gacon G (2011) Septins at the annulus of mammalian sperm. Biol Chem 392:799–803. https://doi.org/10.1515/BC.2011.074
Tu C, Nie H, Meng L et al (2019) Identification of DNAH6 mutations in infertile men with multiple morphological abnormalities of the sperm flagella. Sci Rep 9:15864. https://doi.org/10.1038/s41598-019-52436-7
Urbanska P et al (2018) Ciliary proteins Fap43 and Fap44 interact with each other and are essential for proper cilia and flagella beating. CMLS 75:4479–4493. https://doi.org/10.1007/s00018-018-2819-7
Urbanska P et al (2015) The CSC proteins FAP61 and FAP251 build the basal substructures of radial spoke 3 in cilia. Mol Biol Cell 26:1463–1475. https://doi.org/10.1091/mbc.E14-11-1545
Vadnais ML, Cao W, Aghajanian HK, Haig-Ladewig L, Lin AM, Al-Alao O, Gerton GL (2014) Adenine nucleotide metabolism and a role for AMP in modulating flagellar waveforms in mouse sperm. Biol Reprod. https://doi.org/10.1095/biolreprod.113.114447
van Dam TJP et al (2019) CiliaCarta: an integrated and validated compendium of ciliary genes. PLoS ONE 14:e0216705. https://doi.org/10.1371/journal.pone.0216705
Vincensini L, Blisnick T, Bastin P (2011) 1001 model organisms to study cilia and flagella. Biol Cell 103:109–130. https://doi.org/10.1042/BC20100104
Viswanadha R, Sale WS, Porter ME (2017) Ciliary motility: regulation of axonemal dynein motors. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a018325
Wambergue C et al (2016) Patients with multiple morphological abnormalities of the sperm flagella due to DNAH1 mutations have a good prognosis following intracytoplasmic sperm injection. Hum Reprod 31:1164–1172. https://doi.org/10.1093/humrep/dew083
Wang G et al (2013) In-depth proteomic analysis of the human sperm reveals complex protein compositions. J Proteomics 79:114–122. https://doi.org/10.1016/j.jprot.2012.12.008
Wang W et al (2019) Bi-allelic mutations in CFAP65 lead to severe asthenoteratospermia due to acrosome hypoplasia and flagellum malformations. J Med Genet. https://doi.org/10.1136/jmedgenet-2019-106031
Wang X et al (2017) Homozygous DNAH1 frameshift mutation causes multiple morphological anomalies of the sperm flagella in Chinese. Clin Genet 91:313–321. https://doi.org/10.1111/cge.12857
Wang X et al (2015) Tssk4 is essential for maintaining the structural integrity of sperm flagellum. Mol Hum Reprod 21:136–145. https://doi.org/10.1093/molehr/gau097
Welch EJ, Jones BW, Scott JD (2010) Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv 10:86–97. https://doi.org/10.1124/mi.10.2.6
Whitfield M et al (2019) Mutations in DNAH17, Encoding a sperm-specific axonemal outer dynein arm heavy chain, cause isolated male infertility due to asthenozoospermia. Am J Hum Genet 105:198–212. https://doi.org/10.1016/j.ajhg.2019.04.015
Wu H et al (2019) NovelCFAP43 andCFAP44 mutations cause male infertility with multiple morphological abnormalities of the sperm flagella (MMAF). Reprod Biomed Online 38:769–778. https://doi.org/10.1016/j.rbmo.2018.12.037
Xu Y, Cao J, Huang S, Feng D, Zhang W, Zhu X, Yan X (2015) Characterization of tetratricopeptide repeat-containing proteins critical for cilia formation and function. PLoS ONE 10:e0124378. https://doi.org/10.1371/journal.pone.0124378
Yang P et al (2006) Radial spoke proteins of Chlamydomonas flagella. J Cell Sci 119:1165–1174. https://doi.org/10.1242/jcs.02811
Yang SM, Yang XY, Ding Y, Li H, Wang W, Liu JY, Wen DG (2016) Intracytoplasmic sperm injection outcomes in Chinese men with multiple morphological abnormalities of sperm flagella. Asian J Androl 18:809–811. https://doi.org/10.4103/1008-682X.167722
Young SA, Miyata H, Satouh Y, Aitken RJ, Baker MA, Ikawa M (2016) CABYR is essential for fibrous sheath integrity and progressive motility in mouse spermatozoa. J Cell Sci 129:4379–4387. https://doi.org/10.1242/jcs.193151
Zeytuni N, Zarivach R (2012) Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure 20:397–405. https://doi.org/10.1016/j.str.2012.01.006
Zhang B et al (2019) A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J Exp Med. https://doi.org/10.1084/jem.20182365
Zhang X, Shen Y, Wang X et al (2019) A novel homozygous CFAP65 mutation in humans causes male infertility with multiple morphological abnormalities of the sperm flagella. Clin Genet 96:541–548. https://doi.org/10.1111/cge.13644
Zhang Y et al (2019c) Vertebrate Dynein-f depends on Wdr78 for axonemal localization and is essential for ciliary beat J Mol Cell Biol 11:383–394. https://doi.org/10.1093/jmcb/mjy043
Zhang Y et al (2017) Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice Dev Biol 432:125–139. https://doi.org/10.1016/j.ydbio.2017.09.023
Zhang Y et al (2018) Intraflagellar transporter protein 140 (IFT140), a component of IFT-A complex, is essential for male fertility and spermiogenesis in mice. Cytoskeleton 75:70–84. https://doi.org/10.1002/cm.21427
Zhang Z et al (2016) Intraflagellar transport protein IFT20 is essential for male fertility and spermiogenesis in mice. Mol Biol Cell 1:1. https://doi.org/10.1091/mbc.E16-05-0318
Zhao W, Li Z, Ping P, Wang G, Yuan X, Sun F (2018) Outer dense fibers stabilize the axoneme to maintain sperm motility. J Cell Mol Med 22:1755–1768. https://doi.org/10.1111/jcmm.13457
Zhu X, Liu Y, Yang P (2017) Radial Spokes—a snapshot of the motility regulation, assembly, and evolution of Cilia and Flagella. Cold Spring Harb Perspect Biol 9:a028126. https://doi.org/10.1101/cshperspect.a028126
Acknowledgements
We thank the Cellular Imaging Facility of Institut Cochin (INSERM U1016, CNRS UMR8104, Université Paris Descartes), in particular, Alain Schmitt, Jean-Marc Massé and Azzedine Yacia for electron microscopy procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Touré, A., Martinez, G., Kherraf, ZE. et al. The genetic architecture of morphological abnormalities of the sperm tail. Hum Genet 140, 21–42 (2021). https://doi.org/10.1007/s00439-020-02113-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-020-02113-x