Abstract
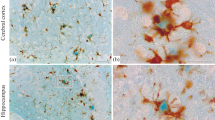
An easy to perform autometallographic technique (AMG) for capturing zinc ions in Alzheimer plaques is presented. The possibility of visualizing loosely bound or free zinc ions in tissue by immersion autometallography (iZnSAMG) is a relatively recent development. The iZnSAMG staining is caused by zinc-sulphur nanocrystals created in 1–2 mm thick brain slices that are immersed in a 0.1% sodium sulphide, 3% glutaraldehyde phosphate buffered solution, the NeoTimm Solution (NTS), for 3 days. When the zinc-sulphur nanocrystals are subsequently silver-enhanced by autometallography, the plaques are readily identified as spheres of dark interlacing strands of different sizes, embedded in the pattern of zinc-enriched terminals. The zinc specificity of the iZnSAMG technique was tested by immersion of brain slides in the chelator DEDTC prior to the NTS immersion. The iZnSAMG detection of zinc ions is easily standardized and can be used in the quantification of plaques with stereological methods. This technique is the first to detect zinc in plaques in the cerebellum of transgenic PS1/APP mice and the first to detect zinc ions in plaques and dystrophic neurites at electron microscopical levels.



Similar content being viewed by others

References
Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS (1997) Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19:939–945
Boutajangout A, Authelet M, Blanchard V, Touchet N, Tremp G, Pradier L, Brion J-P (2004) Characterisation of cytoskeletal abnormalities in mice transgenic for wild-type human tau and familial Alzheimer’s disease mutants of APP and presenilin-1. Neurobiol Dis 15:47–60
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Bush AI (2003) The metallobiology of Alzheimer’s disease. Trends Neurosci 26:207–214
Bush AI, Pettingell WH, Multhaup G, Paradis Md, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE (1994a) Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265:1464–1467
Bush AI, Pettingell WH Jr, Paradis MD, Tanzi RE (1994b) Modulation of A beta adhesiveness and secretase site cleavage by zinc. J Biol Chem 269:12152–12158
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30:665–676
Danscher G (1981) Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry 71:1–16
Danscher G, Haug FM, Fredens K (1973) Effects of didethyldithiocarbamate (DEDTC) on sulphide silver stained boutons. Reversible blocking of Timm’s sulphide silver stain for “heavy” metals in DEDTC treated rats (light microscopy). Exp Brain Res 16:521–532
Danscher G, Jensen KB, Frederickson CJ, Kemp K, Andreasen A, Juhl S, Stoltenberg M, Ravid R (1997a) Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections form autopsy material. J Neurosci Meth 76:53–59
Danscher G, Juhl S, Stoltenberg M, Krunderup B, Schrøder H (1997b) Autometallographic silver amplification of zinc sulphide crystal lattices in zinc enriched synaptic and secretory vesicles. J Histochem Cytochem 45:1503–1510
Danscher G, Stoltenberg M, Bruhn M, Søndergaard C, Jensen D (2004) Immersion autometallography – iZnSAMG: histochemical in situ capturing of zinc ions in catalytic zinc-sulphur nanocrystals. J Histochem Cytochem 52:1619–1625
Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE (1987) A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Meth 20:91–103
Friedlich AL, Lee JY, van Groen T, Cherny RA, Volitakis I, Cole TB, PalmiterRD, Koh JY, Bush AI (2004) Neuronal zinc exchange with the blood vessels wall promotes cerebral amyloid angiopathy in an animal model of Alzheimer’s disease. J Neurosci 24:3453–3459
Howell GA, Welch MG, Frederickson CJ (1984) Stimulation induced uptake and release of zinc in hippocampal slices. Nature 308:736–738
Jaarsma D, Korf J (1990) A novel non-perfusion Timm method for human brain tissue. J Neurosci Meth 35:125–131
Joachim CL, Morris JH, Selkoe DJ (1989) Diffuse senile plaques occur commonly in the cerebellum of Alzheimer’s disease. Am J Pathol 135:309–319
Koh JY, Suh SW, Gwag BJ, HeYY, Hsu CY, Choi DW (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272:1013–1061
Kurt MA, Davies DC, Kidd M, Duff K, Rolph SC, Jennings KH, Howlett DR (2001) Neurodegenerative changes associated with β-amyloid deposition in the brains of mice carrying mutant amyloid precursor pretein and mutant presenilin-1 transgenes. Exp Neurol 171:59–71
Lee JY, Mook-Jung I, Koh JY (1999). Histochemical reactive zinc in plaques of the Swedish mutant β-amyloid precursor protein transgenic mice. J Neurosci 19:RC10:1–5
Liu ST, Howlett G, Barrow CJ (1999) Histidine-13 is a crucial residue in the zinc ion-induced aggregation of the A beta peptide of Alzheimer’s disease. Biochemistry 38:9373–9378
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Pérez-Clausell J, Danscher G (1986) Release of zinc sulphide accumulations into synaptic clefts after in vivo injection of sodium sulphide. Brain Res 362:358–361
Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL (2003) Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting a beta-amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 60:1685–1691
Sasaki A, Shoji M, Harigaya Y, Kawarabayashi T, Ikeda M, Naito M, Matsubara E, Abe K, Nakazato Y (2002) Amyloid cored plaques in PS1/APP transgenic mice are characterized by giant plaques, slightly activated microglia, and the lack of paired helical filament-typed, dystrophic neurites. Virchows Arch 441:358–367
Slomianka L (1992) Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience 48:325–352
Smart TG, Constanti A (1983) Pre- and postsynaptic effects of zinc on in vitro prepyriform neurons. Neurosci Lett 40:205–211
Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ (2000) Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer‘s diseased brains. Brain Res 852:274–278
Takeda A (2001) Zinc homeostasis and functions of zinc in the brain. Biometals 14:343–351
Weiss JH, Hartley DM, Koh JY, Choi DW (1993) AMPA receptor activation potentiates zinc neurotoxicity. Neuron 10:43–49
Zalewski PD, Forbes IJ, Betts WH (1993) Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II). Biochem J 296:403–408
Acknowledgements
We thank Dr. David R. Borchelt, Department of Pathology, The Johns Hopkins University School of Medicine, for kindly providing the transgenic PS1/APP mice. We also gratefully acknowledge the skillful technical assistance of Ms. D. Jensen, Mr. J. Lund, Mr. A. Meier, Ms. H. Mikkelsen, Dr. O. Pletnikova, Ms. M. Sand, and Ms. K. Wiedemann. This study was supported by The Danish Medical Research Council, the Aarhus University Research Foundation, Aase & Ejnar Danielsens Fond, the Danish Medical Association Research Fund (the Søren Segel & Johanne Wiibroe Segels Research Grant), and the Lundbeck, Leo, Beckett, Gangsted, and Novo Nordic Foundations and by a grant from the National Institute of Health (P50 AG 05146).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoltenberg, M., Bruhn, M., Søndergaard, C. et al. Immersion autometallographic tracing of zinc ions in Alzheimer beta-amyloid plaques. Histochem Cell Biol 123, 605–611 (2005). https://doi.org/10.1007/s00418-005-0787-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0787-0