Abstract
Background
The term retinitis pigmentosa (RP) comprises a heterogeneous group of hereditary and sporadic human retinal degenerative diseases. The molecular and cellular events still remain obscure, thus hiding effective therapies. Granulocyte–macrophage colony-stimulating factor (GM-CSF) is a hematopoietic factor which plays a crucial role in protecting neuronal cells. Binding of GM-CSF to its receptor induces several intracellular signaling pathways and kinases. Here we examined whether GM-CSF has a neuroprotective effect on photoreceptor degeneration in Royal College of Surgeons (RCS) rats.
Methods
GM-CSF was injected into the vitreous body of RCS rats either once at the onset of photoreceptor degeneration at day 21, or twice at day 21 and day 42. At day 84, when photoreceptor degeneration is completed, the rats were sacrificed, their eyes enucleated and processed for histological staining and counting the surviving photoreceptor nuclei. The expression of apoptosis-related factors, such as BAD, APAF1 and BCL-2 was examined by Western blot analysis. The expression of neurotrophins such as ciliary neurotrophic factor (CNTF), brain-derived neurotrophic factor (BDNF), and glia-derived neurotrophic actor (GDNF), as well as glial fibrillary acidic protein (GFAP) was analysed by Western blots and immunohistochemistry. The expression of JAK/STAT, ERK1/2 and SRC pathway proteins was assessed by Western blot analysis.
Results
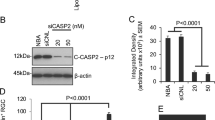
GM-CSF protects significantly against photoreceptor degeneration in comparison to control group. After a single injection of GM-CSF at P21, a 4-fold increase of photoreceptors was observed, whereas eyes which received a repeated injection of GM-CSF at P42 showed a 10-fold increase of photoreceptors. Western blot analysis revealed a decreased BAD and an increased pBAD and BCL-2 expression, indicating changed expression profiles of apoptosis-related proteins. Neurotrophic factors examined are up-regulated, whereas GFAP was also modulated. At cell signalling levels, GM-CSF activates SRC-dependent STAT3 which is independent of JAK2, while proteins of the ERK1/2 pathway are not affected.
Conclusions
The data suggest that GM-CSF is a potent therapeutic agent in photoreceptor degeneration caused by mutation of the receptor tyrosine kinase gene (Mertk), and may be also effective in other photoreceptor degeneration.











Similar content being viewed by others
References
Adler R (1996) Mechanisms of photoreceptor death in retinal degenerations from the cell biology of the 1990s to the ophthalmology of the 21st century? Arch Ophthalmol 114:79–83
Berson EL (1993) Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 34:1659–1676
Pagon RA (1988) Retinitis pigmentosa. Surv Ophthalmol 33:137–177
D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651
Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D (2002) Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem 277:17016–17022
Dowling JE, Sidman RL (1962) Inherited retinal dystrophy in the rat. J Cell Biol 14:73–109
Bok D, Hall MO (1971) The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol 49:664–682
Hall MO (1978) Phagocytosis of light- and dark-adapted rod outer segments by cultured pigment epithelium. Science 202:526–528
LaVail MM, Sidman RL, Gerhardt CO (1975) Congenic strains of RCS rats with inherited retinal dystrophy. J Hered 66:242–244
Mullen RJ, LaVail MM (1976) Inherited retinal dystrophy: Primary defect in pigment epithelium determined with experimental rat chimeras. Science 192:799–801
Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207–211
Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA (2002) Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med 196:135–140
Edwards RB, Szamier RB (1977) Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science 197:1001–1003
Goldman AI, O'Brien PJ (1978) Phagocytosis in the retinal pigment epithelium of the RCS rat. Science 201:1023–1025
Chaitin MH, Hall MO (1983) Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest Ophthalmol Vis Sci 24:812–820
Steinberg RH (1994) Survival factors in retinal degenerations. Curr Opin Neurobiol 4:515–524
Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Peér J (1999) Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retin Eye Res 18:689–735
Chang GQ, Hao Y, Wong F (1993) Apoptosis: Final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron 11:595–605
Katai N, Kikuchi T, Shibuki H, Kuroiwa S, Arai J, Kurokawa T, Yoshimura N (1999) Caspaselike proteases activated in apoptotic photoreceptors of royal college of surgeons rats. Invest Ophthalmol Vis Sci 40:1802–1807
Katai N, Yanagidaira T, Senda N, Murata T, Yoshimura N (2006) Expression of c-jun and bcl-2 family proteins in apoptotic photoreceptors of RCS rats. Jpn J Ophthalmol 50:121–127
Nickells RW, Zack DJ (1996) Apoptosis in ocular disease: A molecular overview. Ophthalmic Genet 17:145–165
Marc RE, Jones BW (2003) Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol 28:139–147
Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D (2000) Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet 26:270–271
McHenry CL, Liu Y, Feng W, Nair AR, Feathers KL, Ding X, Gal A, Vollrath D, Sieving PA, Thompson DA (2004) MERTK arginine-844-cysteine in a patient with severe rod-cone dystrophy: loss of mutant protein function in transfected cells. Invest Ophthalmol Vis Sci 45:1456–1463
Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, Vollrath D (2003) An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci 44:826–838
LaVail MM (2001) Legacy of the RCS rat: Impact of a seminal study on retinal cell biology and retinal degenerative diseases. Prog Brain Res 131:617–627
Chader GJ (2002) Animal models in research on retinal degenerations: Past progress and future hope. Vision Res 42:393–399
Vollrath D, Feng W, Duncan JL (2001) Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of mertk. Proc Natl Acad Sci USA 98:12584–12589
Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM (1992) Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci 12:3554–3567
Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM (1990) Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 347:83–86
LaVail MM, Yasumura D, Matthes MT (1998) Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci 39:592–602
Machida S, Tanaka M, Ishii T, Ohtaka K, Takahashi T, Tazawa Y (2004) Neuroprotective effect of hepatocyte growth factor against photoreceptor degeneration in rats. Invest Ophthalmol Vis Sci 45:4174–4182
Whiteley SJ, Klassen H, Coffey PJ, Young MJ (2001) Photoreceptor rescue after low-dose intravitreal IL-1beta injection in the RCS rat. Exp Eye Res 73:557–568
Perry J, Du J, Kjeldbye H, Gouras P (1995) The effects of bFGF on RCS rat eyes. Curr Eye Res 14:585–592
Kent TL, Glybina IV, Abrams GW, Iezzi R (2008) Chronic intravitreous infusion of ciliary neurotrophic factor modulates electrical retinal stimulation thresholds in the RCS rat. Invest Ophthalmol Vis Sci 49:372–379
Frasson M, Picaud S, Leveillard T, Simonutti M, Mohand-Said S, Dreyfus H, Hicks D, Sabel J (1999) Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci 40:2724–2734
Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Sakamoto T, Tabata T, Ueda Y, Hasegawa M, Tobimatsu S, Ishibashi T, Sueishi K (2003) Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther 10:1503–1511
Cayouette M, Behn D, Sendtner M, Lachapelle P, Gravel C (1998) Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci 18:9282–9293
Tao W, Wen R, Goddard MB, Sherman SD, O'Rourke PJ, Stabila PF, Bell WJ, Dean BJ, Kauper KA, Budz VA, Tsiaras WG, Acland GM, Pearce-Kelling S, Laties AM, Aguirre GD (2002) Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci 43:3292–3298
Chaum E (2003) Retinal neuroprotection by growth factors: a mechanistic perspective. J Cell Biochem 88:57–75
McGee Sanftner LH, Abel H, Hauswirth WW, Flannery JG (2001) Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther 4:622–629
Dame JB, Christensen RD, Juul SE (1999) The distribution of granulocyte–macrophage colony-stimulating factor and its receptor in the developing human fetus. Pediatr Res 46:358–366
Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schäbitz WR (2005) The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 115:2083–2098
Schabitz WR, Kruger C, Pitzer C, Weber D, Laage R, Gassler N, Aronowski J, Mier W, Kirsch F, Dittgen T, Bach A, Sommer C, Schneider A (2008) A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J Cereb Blood Flow Metab 28:29–43
Metcalf D (1989) The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature 339:27–30
Nicola NA (2001) GM-CSF receptor. In: Oppenheim JJ, Feldman M (eds) Cytokine reference. Academic Press, San Diego, pp 1935–1944
Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ (1995) Bcl-XL and bcl-2 repress a common pathway of cell death. J Exp Med 182:821–828
Peterson WM, Wang Q, Tzekova R, Wiegand SJ (2000) Ciliary neurotrophic factor and stress stimuli activate the jak-STAT pathway in retinal neurons and glia. J Neurosci 20:4081–4090
Choi JK, Choi BH, Ha Y, Park H, Yoon SH, Park HC, Park SR (2007) Signal transduction pathways of GM-CSF in neural cell lines. Neurosci Lett 420:217–222
Dahl ME, Arai KI, Watanabe S (2000) Association of lyn tyrosine kinase to the GM-CSF and IL-3 receptor common betac subunit and role of src tyrosine kinases in DNA synthesis and anti-apoptosis. Genes Cells 5:143–153
Schallenberg M, Charalambous P, Thanos S (2009) GM-CSF regulates the ERK1/2 pathways and protects injured retinal ganglion cells from induced death. Exp Eye Res 89:665–677
Armitage JO (1998) Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 92:4491–4508
Raivich G, Gehrmann J, Kreutzberg GW (1991) Increase of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor receptors in the regenerating rat facial nucleus. J Neurosci Res 30:682–686
Moore S, Thanos S (1996) The concept of microglia in relation to central nervous system disease and regeneration. Prog Neurobiol 48:441–460
Liva SM, Kahn MA, Dopp JM, de Vellis J (1999) Signal transduction pathways induced by GM-CSF in microglia: significance in the control of proliferation. Glia 26:344–352
David S, Ousman SS (2002) Recruiting the immune response to promote axon regeneration in the injured spinal cord. Neuroscientist 8:33–41
Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS (1997) Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 3:73–76
Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, Park HS (2005) Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng 11:913–922
Ha Y, Kim YS, Cho JM, Yoon SH, Park SR, Yoon DH, Kim EY, Park HC (2005) Role of granulocyte–macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J Neurosurg Spine 2:55–61
Kim JK, Choi BH, Park HC, Park SR, Kim YS, Yoon SH, Park HS, Kim EY, Ha Y (2004) Effects of GM-CSF on the neural progenitor cells. Neuroreport 15:2161–2165
Lawrence JM, Keegan DJ, Muir EM, Coffey PJ, Rogers JH, Wilby MJ, Fawcett JW, Lund RD (2004) Transplantation of schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons rats. Invest Ophthalmol Vis Sci 45:267–274
Tso MO, Zhang C, Abler AS, Chang CJ, Wong F, Chang GQ, Lam TT (1994) Apoptosis leads to photoreceptor degeneration in inherited retinal dystrophy of RCS rats. Invest Ophthalmol Vis Sci 35:2693–2699
Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, Seeley RJ (2005) GM-CSF action in the CNS decreases food intake and body weight. J Clin Invest 115:3035–3044
Huang X, Choi JK, Park SR, Ha Y, Park H, Yoon SH, Park HC, Park JO, Choi BH (2007) GM-CSF inhibits apoptosis of neural cells via regulating the expression of apoptosis-related proteins. Neurosci Res 58:50–57
Englund-Johansson U, Mohlin C, Liljekvist-Soltic I, Ekström P, Johansson K (2010) Human neural progenitor cells promote photoreceptor survival in retinal explants. Exp Eye Res 90:292–299
Rickman DW, Nacke RE, Bowes Rickman C (1999) Characterization of the cell death promoter, bad, in the developing rat retina and forebrain. Brain Res Dev Brain Res 115:41–47
Chen J, Flannery JG, LaVail MM, Steinberg RH, Xu J, Simon MI (1996) Bcl-2 overexpression reduces apoptotic photoreceptor cell death in three different retinal degenerations. Proc Natl Acad Sci USA 93:7042–7047
McKernan DP, Cotter TG (2007) A critical role for bim in retinal ganglion cell death. J Neurochem 102:922–930
Battle TE, Frank DA (2002) The role of STATs in apoptosis. Curr Mol Med 2:381–392
Song Y, Zhao L, Tao W, Laties AM, Luo Z, Wen R (2003) Photoreceptor protection by cardiotrophin-1 in transgenic rats with the rhodopsin mutation s334ter. Invest Ophthalmol Vis Sci 44:4069–4075
Ihle JN (1996) STATs: Signal transducers and activators of transcription. Cell 84:331–334
O'Shea JJ (1997) Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity 7:1–11
Darnell JE Jr (1997) STATs and gene regulation. Science 277:1630–1635
Watanabe S, Itoh T, Arai K (1996) JAK2 is essential for activation of c-fos and c-myc promoters and cell proliferation through the human granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. J Biol Chem 271:12681–12686
Quelle FW, Sato N, Witthuhn BA, Inhorn RC, Eder M, Miyajima A, Griffin JD, Ihle JN (1994) JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol 14:4335–4341
Samardzija M, Wenzel A, Aufenberg S, Thiersch M, Reme C, Grimm C (2006) Differential role of jak-STAT signaling in retinal degenerations. FASEB J 20:2411–2413
Han C, Bowen WC, Michalopoulos GK, Wu T (2008) Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of src and epidermal growth factor receptor (EGFR) in hepatocytes. J Cell Physiol 216:486–497
He JC, Gomes I, Nguyen T, Jayaram G, Ram PT, Devi LA, Iyengar R (2005) The G alpha(o/i)-coupled cannabinoid receptor-mediated neurite outgrowth involves rap regulation of src and Stat3. J Biol Chem 280:33426–33434
Alonso G, Koegl M, Mazurenko N, Courtneidge SA (1995) Sequence requirements for binding of src family tyrosine kinases to activated growth factor receptors. J Biol Chem 270:9840–9848
Anderson SM, Jorgensen B (1995) Activation of src-related tyrosine kinases by IL-3. J Immunol 155:1660–1670
Weng G, Bhalla US, Iyengar R (1999) Complexity in biological signaling systems. Science 284:92–96
Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR (2009) CNTF induces photoreceptor neuroprotection and Müller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res 88:1051–1064
Touchard E, Heiduschka P, Berdugo M, Kowalczuk L, Bigey P, Chahory S, Gandolphe C, Jeanny JC, Behar-Cohen F (2011) Non-viral gene therapy for GDNF production in RCS rat: the crucial role of the plasmid dose. Gene Ther, doi:10.1038/gt.2011.154
Zhang M, Mo X, Fang Y, Guo W, Wu J, Zhang S, Huang Q (2009) Rescue of photoreceptors by BDNF gene transfer using in vivo electroporation in the RCS rat of retinitis pigmentosa. Curr Eye Res 34:791–799
Bouhy D, Malgrange B, Multon S, Poirrier AL, Scholtes F, Schoenen J, Franzen R (2006) Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J 20:1239–1241
Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K (2002) Microglia–Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci 22:9228–9236
Joly S, Pernet V, Chemtob S, Di Polo A, Lachapelle P (2007) Neuroprotection in the juvenile rat model of light-induced retinopathy: Evidence suggesting a role for FGF-2 and CNTF. Invest Ophthalmol Vis Sci 48:2311–2320
Valter K, Bisti S, Stone J (2003) Location of CNTFRalpha on outer segments: Evidence of the site of action of CNTF in rat retina. Brain Res 985:169–175
Cuthbertson RA, Lang RA, Coghlan JP (1990) Macrophage products IL-1 alpha, TNF alpha and bFGF may mediate multiple cytopathic effects in the developing eyes of GM-CSF transgenic mice. Exp Eye Res 51:335–344
Rhee KD, Ruiz A, Duncan JL, Hauswirth WW, Lavail MM, Bok D, Yang XJ (2007) Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 48:1389–1400
Acknowledgements
The authors thank M. Wissing and M. Langkamp-Flock for their technical assistance. The work was supported by the Deutsche Forschungsgemeinschaft (DFG Grants Th386/16-1 and Th386/16-2 to S.T.) and the IMF-Münster (Grant NA 110503 to S.T.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schallenberg, M., Charalambous, P. & Thanos, S. GM-CSF protects rat photoreceptors from death by activating the SRC-dependent signalling and elevating anti-apoptotic factors and neurotrophins. Graefes Arch Clin Exp Ophthalmol 250, 699–712 (2012). https://doi.org/10.1007/s00417-012-1932-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-1932-9