Abstract
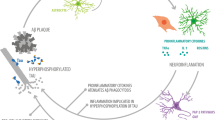
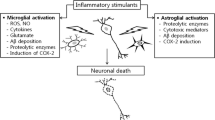
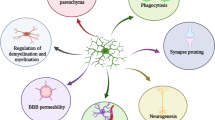
Alzheimer´s disease (AD) stands out as the most common chronic neurodegenerative disorder. AD is characterized by progressive cognitive decline and memory loss, with neurodegeneration as its primary pathological feature. The role of neuroinflammation in the disease course has become a focus of intense research. While microglia, the brain’s resident macrophages, have been pivotal to study central immune inflammation, recent evidence underscores the contributions of other cellular entities to the neuroinflammatory process. In this article, we review the inflammatory role of microglia and astrocytes, focusing on their interactions with AD’s core pathologies, amyloid beta deposition, and tau tangle formation. Additionally, we also discuss how different modes of regulated cell death in AD may impact the chronic neuroinflammatory environment. This review aims to highlight the evolving landscape of neuroinflammatory research in AD and underscores the importance of considering multiple cellular contributors when developing new therapeutic strategies.



Similar content being viewed by others
Abbreviations
- ACT:
-
Antichymotrypsin
- AD:
-
Alzheimer disease
- Aβ:
-
Amyloid-β
- APOE:
-
Apolipoprotein E
- ApoJ/Clusterin:
-
Apolipoprotein J
- ALS:
-
Amyotrophic lateral sclerosis
- ASC:
-
Apoptosis-associated Speck-like protein containing a CARD
- APP:
-
Amyloid precursor protein
- BAMs:
-
Border-associated macrophages
- BBB:
-
Blood–brain barrier
- Bcl:
-
B-cell lymphoma
- BECs:
-
Brain endothelial cells
- C:
-
Complement component
- C1q:
-
Subcomponent q
- CCL:
-
C–C motif chemokine ligand
- CD40L:
-
CD40 ligand
- CDK:
-
Cyclin-dependent kinase
- CNS:
-
Central nervous system
- CN:
-
Calcineurin
- COX:
-
Cyclooxygenase
- DAMPs:
-
Damage-associated molecular patterns
- EOAD:
-
Early onset familial alzheimer disease
- FERMT:
-
Fermitin family homolog
- GFAP:
-
Glial fibrillary acidic protein
- GMF:
-
Glia maturation factor
- GBP:
-
Guanylate binding protein
- GSK:
-
Glycogen synthase kinase
- GWAS:
-
Genome-wide association studies
- HSPs:
-
Heat shock proteins
- HSPGs:
-
Heparan sulfate proteoglycans
- HMGB:
-
High mobility group box
- ICAM-1:
-
Intercellular adhesion molecule 1
- IDE:
-
Insulin-degrading enzyme
- IFN:
-
Interferon
- IL:
-
Interleukin
- iPS:
-
Induced pluripotent stem
- JAK:
-
Janus kinase
- MAPK:
-
Mitogen-activated protein kinase
- MMP9:
-
Matrix metalloprotease 9
- MLKL:
-
Mixed lineage kinase domain-like
- MMP:
-
Matrix metalloproteinase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NDDs:
-
Neurodegenerative diseases
- NEP:
-
Neprylisin
- NFAT:
-
Nuclear factor of activated T cells
- NFκB:
-
Nuclear factor-κB
- NLRs:
-
Nucleotide-binding oligomerization domain-like receptors
- NFTs:
-
Neurofibrillary tangles
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NOD:
-
Nucleotide-binding oligomerization domain
- PAR:
-
Protease-activated receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- PD:
-
Parkinson’s disease
- PDGFRβ:
-
Platelet-derived growth factor receptor β
- PG:
-
Prostaglandin
- PKs:
-
Protein kinases
- PFFs:
-
Preformed fibrils
- PRRs:
-
Pattern recognition receptors
- PSEN:
-
Presenilin
- PVMs:
-
Perivascular macrophages
- RIPK:
-
Receptor-interactive protein kinases
- RAGE:
-
Receptor for advanced glycation end products
- RB:
-
Retinoblastoma
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- RCD:
-
Regulated cell death
- SAM:
-
Senescent-associated microglia
- SASP:
-
Senescence-associated secretory phenotype
- SAHF:
-
Senescence-associated heterochromatin foci
- SORL:
-
Sortilin-related receptor
- SP:
-
Substance P
- STAT:
-
Signal transducer and activator of transcription
- SRs:
-
Scavenger receptors
- TNTs:
-
Tunnelling nanotubes
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TREM:
-
Triggering receptor expressed on myeloid cells
- TGF:
-
Transforming growth factor
- TLRs:
-
Toll-like receptors
- VEGF:
-
Vascular endothelial growth factor
References
Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM et al (2009) Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci Off J Soc Neurosci 29:12957–12969. https://doi.org/10.1523/JNEUROSCI.1064-09.2009
Acosta C, Anderson HD, Anderson CM (2017) Astrocyte dysfunction in Alzheimer disease. J Neurosci Res 95:2430–2447. https://doi.org/10.1002/jnr.24075
Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP et al (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15:978–990. https://doi.org/10.1038/ncb2784
Alonso AC, Grundke-Iqbal I, Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 2:783–787. https://doi.org/10.1038/nm0796-783
Alzheimer A (1907) Uber eine eigenartige Erkrankung der Hirnrinde. Zentralbl Nervenh Psych 18:177–179
Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR (1995) An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde.” Clin Anat N Y N 8:429–431. https://doi.org/10.1002/ca.980080612
Andersen JV, Skotte NH, Christensen SK, Polli FS, Shabani M, Markussen KH et al (2021) Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Death Dis 12:1–13. https://doi.org/10.1038/s41419-021-04237-y
Arendt T, Stieler J, Strijkstra AM, Hut RA, Rüdiger J, Van der Zee EA et al (2003) Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci Off J Soc Neurosci 23:6972–6981. https://doi.org/10.1523/JNEUROSCI.23-18-06972.2003
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T et al (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18:1584–1593. https://doi.org/10.1038/nn.4132
Avila-Muñoz E, Arias C (2014) When astrocytes become harmful: functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res Rev 18:29–40. https://doi.org/10.1016/j.arr.2014.07.004
Basak JM, Verghese PB, Yoon H, Kim J, Holtzman DM (2012) Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of Aβ uptake and degradation by astrocytes. J Biol Chem 287:13959–13971. https://doi.org/10.1074/jbc.M111.288746
Basheer N, Smolek T, Hassan I, Liu F, Iqbal K, Zilka N et al (2023) Does modulation of tau hyperphosphorylation represent a reasonable therapeutic strategy for Alzheimer’s disease? From preclinical studies to the clinical trials. Mol Psychiatry 28:2197–2214. https://doi.org/10.1038/s41380-023-02113-z
Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC et al (2012) Clinical and biomarker changes in dominantly inherited alzheimer’s disease. N Engl J Med 367:795–804. https://doi.org/10.1056/NEJMoa1202753
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R et al (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427
Bellaver B, Povala G, Ferreira PCL, Ferrari-Souza JP, Leffa DT, Lussier FZ et al (2023) Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer’s disease. Nat Med 29:1775–1781. https://doi.org/10.1038/s41591-023-02380-x
Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N et al (2022) New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet 54:412–436. https://doi.org/10.1038/s41588-022-01024-z
Berberich I, Shu GL, Clark EA (1994) Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J Immunol 153:4357–4366. https://doi.org/10.4049/jimmunol.153.10.4357
Bettcher BM, Tansey MG, Dorothée G, Heneka MT (2021) Peripheral and central immune system crosstalk in Alzheimer disease—a research prospectus. Nat Rev Neurol 17:689–701. https://doi.org/10.1038/s41582-021-00549-x
Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT (2010) Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68:19–31. https://doi.org/10.1016/j.neuron.2010.08.023
Bhusal A, Afridi R, Lee W-H, Suk K (2023) Bidirectional communication between microglia and astrocytes in neuroinflammation. Curr Neuropharmacol 21:2020–2029. https://doi.org/10.2174/1570159X21666221129121715
Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G et al (2006) Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312:1389–1392. https://doi.org/10.1126/science.1123511
Bolós M, Llorens-Martín M, Jurado-Arjona J, Hernández F, Rábano A, Avila J (2016) Direct evidence of internalization of tau by microglia in vitro and in vivo. J Alzheimers Dis JAD 50:77–87. https://doi.org/10.3233/JAD-150704
Borst K, Dumas AA, Prinz M (2021) Microglia: immune and non-immune functions. Immunity 54:2194–2208. https://doi.org/10.1016/j.immuni.2021.09.014
Bouvier DS, Jones EV, Quesseveur G, Davoli MA, Ferreira AT, Quirion R et al (2016) High resolution dissection of reactive glial nets in Alzheimer’s disease. Sci Rep 6:24544. https://doi.org/10.1038/srep24544
Brelstaff JH, Mason M, Katsinelos T, McEwan WA, Ghetti B, Tolkovsky AM et al (2021) Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci Adv 7:eabg4980. https://doi.org/10.1126/sciadv.abg4980
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 3:186–191. https://doi.org/10.1016/j.jalz.2007.04.381
Brosseron F, Krauthausen M, Kummer M, Heneka MT (2014) Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol 50:534–544. https://doi.org/10.1007/s12035-014-8657-1
Buckley MW, McGavern DB (2022) Immune dynamics in the CNS and its barriers during homeostasis and disease. Immunol Rev 306:58–75. https://doi.org/10.1111/imr.13066
Burgaletto C, Munafò A, Di Benedetto G, De Francisci C, Caraci F, Di Mauro R et al (2020) The immune system on the TRAIL of Alzheimer’s disease. J Neuroinflammation 17:298. https://doi.org/10.1186/s12974-020-01968-1
Burini RC, Anderson E, Durstine JL, Carson JA (2020) Inflammation, physical activity, and chronic disease: an evolutionary perspective. Sports Med Health Sci 2:1–6. https://doi.org/10.1016/j.smhs.2020.03.004
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN et al (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308. https://doi.org/10.1016/S0896-6273(00)80781-3
Butovsky O, Weiner HL (2018) Microglial signatures and their role in health and disease. Nat Rev Neurosci 19:622–635. https://doi.org/10.1038/s41583-018-0057-5
Caccamo A, Branca C, Piras IS, Ferreira E, Huentelman MJ, Liang WS et al (2017) Necroptosis activation in Alzheimer’s disease. Nat Neurosci 20:1236–1246. https://doi.org/10.1038/nn.4608
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM et al (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9:917–924. https://doi.org/10.1038/nn1715
Casey CS, Atagi Y, Yamazaki Y, Shinohara M, Tachibana M, Fu Y et al (2015) Apolipoprotein E inhibits cerebrovascular pericyte mobility through a RhoA protein-mediated pathway*. J Biol Chem 290:14208–14217. https://doi.org/10.1074/jbc.M114.625251
Castellani G, Croese T, Peralta Ramos JM, Schwartz M (2023) Transforming the understanding of brain immunity. Science 380:eabo7649. https://doi.org/10.1126/science.abo7649
Chai YL, Lee JH, Chong JR, Ballard C, Francis PT, Kennedy BK et al (2023) Inflammatory panel cytokines are elevated in the neocortex of late-stage Alzheimer’s disease but not Lewy body dementias. J Neuroinflammation 20:111. https://doi.org/10.1186/s12974-023-02789-8
Chakraborty R, Nonaka T, Hasegawa M, Zurzolo C (2023) Tunnelling nanotubes between neuronal and microglial cells allow bi-directional transfer of α-Synuclein and mitochondria. Cell Death Dis 14:1–12. https://doi.org/10.1038/s41419-023-05835-8
Chen X, Deng S, Wang W, Castiglione S, Duan Z, Luo L et al (2022) Human antimicrobial peptide LL-37 contributes to Alzheimer’s disease progression. Mol Psychiatry 27:4790–4799. https://doi.org/10.1038/s41380-022-01790-6
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. https://doi.org/10.1186/1742-2094-11-98
Chevriaux A, Pilot T, Derangère V, Simonin H, Martine P, Chalmin F et al (2020) Cathepsin B is required for NLRP3 inflammasome activation in macrophages, through NLRP3 interaction. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.00167
Chun H, Im H, Kang YJ, Kim Y, Shin JH, Won W et al (2020) Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2- production. Nat Neurosci 23:1555–1566. https://doi.org/10.1038/s41593-020-00735-y
Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G et al (1997) Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat Med 3:67–72. https://doi.org/10.1038/nm0197-67
Colombo E, Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37:608–620. https://doi.org/10.1016/j.it.2016.06.006
Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Núñez G et al (2017) Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci 114:E961–E969. https://doi.org/10.1073/pnas.1613305114
Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ et al (2010) YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiatry 68:903–912. https://doi.org/10.1016/j.biopsych.2010.08.025
Crols R, Saerens J, Noppe M, Lowenthal A (1986) Increased GFAp levels in CSF as a marker of organicity in patients with Alzheimer’s disease and other types of irreversible chronic organic brain syndrome. J Neurol 233:157–160. https://doi.org/10.1007/BF00314423
Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ (1996) Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett 203:17–20. https://doi.org/10.1016/0304-3940(95)12252-4
Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT (2011) Age-related alterations in the dynamic behavior of microglia. Aging Cell 10:263–276. https://doi.org/10.1111/j.1474-9726.2010.00660.x
Davis N, Mota BC, Stead L, Palmer EOC, Lombardero L, Rodríguez-Puertas R et al (2021) Pharmacological ablation of astrocytes reduces Aβ degradation and synaptic connectivity in an ex vivo model of Alzheimer’s disease. J Neuroinflammation 18:73. https://doi.org/10.1186/s12974-021-02117-y
De Kimpe L, van Haastert ES, Kaminari A, Zwart R, Rutjes H, Hoozemans JJM et al (2013) Intracellular accumulation of aggregated pyroglutamate amyloid beta: convergence of aging and Aβ pathology at the lysosome. Age Dordr Neth 35:673–687. https://doi.org/10.1007/s11357-012-9403-0
De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W et al (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391:387–390. https://doi.org/10.1038/34910
Dean DC III, Hurley SA, Kecskemeti SR, O’Grady JP, Canda C, Davenport-Sis NJ et al (2017) Association of amyloid pathology with myelin alteration in preclinical alzheimer disease. JAMA Neurol 74:41–49. https://doi.org/10.1001/jamaneurol.2016.3232
Depp C, Sun T, Sasmita AO, Spieth L, Berghoff SA, Nazarenko T et al (2023) Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature 618:349–357. https://doi.org/10.1038/s41586-023-06120-6
Dermitzakis I, Theotokis P, Evangelidis P, Delilampou E, Evangelidis N, Chatzisavvidou A et al (2023) CNS border-associated macrophages: ontogeny and potential implication in disease. Curr Issues Mol Biol 45:4285–4300. https://doi.org/10.3390/cimb45050272
DiPatre PL, Gelman BB (1997) Microglial cell activation in aging and Alzheimer disease: partial linkage with neurofibrillary tangle burden in the hippocampus. J Neuropathol Exp Neurol 56:143–149. https://doi.org/10.1097/00005072-199702000-00004
DiSabato DJ, Quan N, Godbout JP (2016) Neuroinflammation: the devil is in the details. J Neurochem 139(Suppl 2):136–153. https://doi.org/10.1111/jnc.13607
Dixit R, Ross JL, Goldman YE, Holzbaur ELF (2008) Differential regulation of dynein and kinesin motor proteins by tau. Science 319:1086–1089. https://doi.org/10.1126/science.1152993
Domingues C, da Cruz E, Silva OAB, Henriques AG (2017) Impact of cytokines and chemokines on alzheimer’s disease neuropathological hallmarks. Curr Alzheimer Res 14:870–882. https://doi.org/10.2174/1567205014666170317113606
Ekonomou A, Savva GM, Brayne C, Forster G, Francis PT, Johnson M et al (2015) Stage-specific changes in neurogenic and glial markers in Alzheimer’s disease. Biol Psychiatry 77:711–719. https://doi.org/10.1016/j.biopsych.2014.05.021
El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD (1996) Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature 382:716–719. https://doi.org/10.1038/382716a0
Eltom K, Mothes T, Libard S, Ingelsson M, Erlandsson A (2024) Astrocytic accumulation of tau fibrils isolated from Alzheimer’s disease brains induces inflammation, cell-to-cell propagation and neuronal impairment. Acta Neuropathol Commun 12:34. https://doi.org/10.1186/s40478-024-01745-8
Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC et al (2004) Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 61:668–672. https://doi.org/10.1001/archneur.61.5.668
Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24:312–325. https://doi.org/10.1038/s41593-020-00783-4
Fancy NN, Smith AM, Caramello A, Tsartsalis S, Davey K, Muirhead RCJ et al (2024) Characterisation of premature cell senescence in Alzheimer’s disease using single nuclear transcriptomics. Acta Neuropathol (Berl) 147:78. https://doi.org/10.1007/s00401-024-02727-9
Fassbender K, Walter S, Kühl S, Landmann R, Ishii K, Bertsch T et al (2004) The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J Off Publ Fed Am Soc Exp Biol 18:203–205. https://doi.org/10.1096/fj.03-0364fje
Fleeman RM, Proctor EA (2021) Astrocytic propagation of tau in the context of alzheimer’s disease. Front Cell Neurosci 15:645233. https://doi.org/10.3389/fncel.2021.645233
Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G et al (2014) ASC has extracellular and prionoid activities that propagate inflammation. Nat Immunol 15:727–737. https://doi.org/10.1038/ni.2913
Friker LL, Scheiblich H, Hochheiser IV, Brinkschulte R, Riedel D, Latz E et al (2020) β-Amyloid clustering around ASC fibrils boosts its toxicity in microglia. Cell Rep 30:3743-3754.e6. https://doi.org/10.1016/j.celrep.2020.02.025
Fu H, Hardy J, Duff KE (2018) Selective vulnerability in neurodegenerative diseases. Nat Neurosci 21:1350–1358. https://doi.org/10.1038/s41593-018-0221-2
Füger P, Hefendehl JK, Veeraraghavalu K, Wendeln A-C, Schlosser C, Obermüller U et al (2017) Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat Neurosci 20:1371–1376. https://doi.org/10.1038/nn.4631
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C et al (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25:1822–1832. https://doi.org/10.1038/s41591-019-0675-0
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P et al (2018) Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 25:486–541. https://doi.org/10.1038/s41418-017-0012-4
Gaminde-Blasco A, Senovilla-Ganzo R, Balantzategi U, García-Moreno F, Matute C, Baleriola J et al (2024) Amyloid-β increases MBP and MOBP translation in oligodendrocytes through dysregulation of hnRNP A2 dependent RNA dynamics. BioRxiv. https://doi.org/10.1101/2024.04.19.590214
Garbuz DG, Zatsepina OG, Evgenev MB (2021) Beta Amyloid, tau protein, and neuroinflammation: an attempt to integrate different hypotheses of alzheimer’s disease pathogenesis. Mol Biol 55:670–682. https://doi.org/10.1134/S002689332104004X
Garnier-Crussard A, Bougacha S, Wirth M, Dautricourt S, Sherif S, Landeau B et al (2022) White matter hyperintensity topography in Alzheimer’s disease and links to cognition. Alzheimers Dement 18:422–433. https://doi.org/10.1002/alz.12410
Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J et al (2020) Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577:399–404. https://doi.org/10.1038/s41586-019-1895-7
Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA et al (2013) Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an alzheimer’s mouse model. J Neurosci 33:5053–5064. https://doi.org/10.1523/JNEUROSCI.4361-12.2013
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845. https://doi.org/10.1126/science.1194637
Giusti V, Kaur G, Giusto E, Civiero L (2024) Brain clearance of protein aggregates: a close-up on astrocytes. Mol Neurodegener 19:5. https://doi.org/10.1186/s13024-024-00703-1
Gonzales MM, Garbarino VR, Marques Zilli E, Petersen RC, Kirkland JL, Tchkonia T et al (2022) Senolytic therapy to modulate the progression of alzheimer’s disease (SToMP-AD): a pilot clinical trial. J Prev Alzheimers Dis 9:22–29. https://doi.org/10.14283/jpad.2021.62
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C et al (2019) Cellular senescence: defining a path forward. Cell 179:813–827. https://doi.org/10.1016/j.cell.2019.10.005
Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L (2014) Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB Signaling pathway and proinflammatory response in human pericytes. J Biol Chem 289:2457–2468. https://doi.org/10.1074/jbc.M113.521161
Haage V, De Jager PL (2022) Neuroimmune contributions to Alzheimer’s disease: a focus on human data. Mol Psychiatry 27:3164–3181. https://doi.org/10.1038/s41380-022-01637-0
Habib N, McCabe C, Medina S, Varshavsky M, Kitsberg D, Dvir-Szternfeld R et al (2020) Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci 23:701–706. https://doi.org/10.1038/s41593-020-0624-8
Haim LB, Ceyzériat K, Sauvage MAC, Aubry F, Auregan G, Guillermier M et al (2015) The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in alzheimer’s and huntington’s diseases. J Neurosci 35:2817–2829. https://doi.org/10.1523/JNEUROSCI.3516-14.2015
Han C, Yang Y, Guan Q, Zhang X, Shen H, Sheng Y et al (2020) New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. J Cell Mol Med 24:8078–8090. https://doi.org/10.1111/jcmm.15439
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185. https://doi.org/10.1126/science.1566067
Hardy S (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. https://doi.org/10.1126/science.1072994
Hasel P, Rose IVL, Sadick JS, Kim RD, Liddelow SA (2021) Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci 24:1475–1487. https://doi.org/10.1038/s41593-021-00905-6
Hecimovic S, Wang J, Dolios G, Martinez M, Wang R, Goate AM (2004) Mutations in APP have independent effects on Aβ and CTFγ generation. Neurobiol Dis 17:205–218. https://doi.org/10.1016/j.nbd.2004.04.018
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477. https://doi.org/10.1038/nri3705
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678. https://doi.org/10.1038/nature11729
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621. https://doi.org/10.1038/s41583-018-0055-7
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T et al (2005) Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation 2:22. https://doi.org/10.1186/1742-2094-2-22
Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P et al (2016) Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener 11:3. https://doi.org/10.1186/s13024-016-0071-x
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci Off J Soc Neurosci 28:8354–8360. https://doi.org/10.1523/JNEUROSCI.0616-08.2008
Hollville E, Romero SE, Deshmukh M (2019) Apoptotic cell death regulation in neurons. FEBS J 286:3276–3298. https://doi.org/10.1111/febs.14970
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL et al (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15:565–581. https://doi.org/10.1038/s41582-019-0244-7
Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS et al (1986) Defective brain microtubule assembly in Alzheimer’s disease. Lancet Lond Engl 2:421–426. https://doi.org/10.1016/s0140-6736(86)92134-3
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A et al (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575:669–673. https://doi.org/10.1038/s41586-019-1769-z
Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24:173–182. https://doi.org/10.1016/0165-5728(89)90115-X
Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ et al (1999) Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 52:1397–1403. https://doi.org/10.1212/wnl.52.7.1397
Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ et al (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313:1924–1938. https://doi.org/10.1001/jama.2015.4668
Jellinger KA (2010) Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med 14:457–487. https://doi.org/10.1111/j.1582-4934.2010.01010.x
Jiang S, Maphis NM, Binder J, Chisholm D, Weston L, Duran W et al (2021) Proteopathic tau primes and activates interleukin-1β via myeloid-cell-specific MyD88- and NLRP3-ASC-inflammasome pathway. Cell Rep 36:109720. https://doi.org/10.1016/j.celrep.2021.109720
Jones VC, Atkinson-Dell R, Verkhratsky A, Mohamet L (2017) Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis 8:e2696. https://doi.org/10.1038/cddis.2017.89
Jorfi M, Park J, Hall CK, Lin C-CJ, Chen M, von Maydell D et al (2023) Infiltrating CD8+ T cells exacerbate Alzheimer’s disease pathology in a 3D human neuroimmune axis model. Nat Neurosci 26:1489–1504. https://doi.org/10.1038/s41593-023-01415-3
Karran E, De Strooper B (2022) The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov 21:306–318. https://doi.org/10.1038/s41573-022-00391-w
Katsouri L, Birch AM, Renziehausen AWJ, Zach C, Aman Y, Steeds H et al (2020) Ablation of reactive astrocytes exacerbates disease pathology in a model of Alzheimer’s disease. Glia 68:1017–1030. https://doi.org/10.1002/glia.23759
Kim H, Leng K, Park J, Sorets AG, Kim S, Shostak A et al (2022) Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin. Nat Commun 13:6581. https://doi.org/10.1038/s41467-022-34412-4
Kim J, Yoo ID, Lim J, Moon J-S (2024) Pathological phenotypes of astrocytes in Alzheimer’s disease. Exp Mol Med 56:95–99. https://doi.org/10.1038/s12276-023-01148-0
Kim J-H, Afridi R, Han J, Jung H-G, Kim S-C, Hwang EM et al (2021) Gamma subunit of complement component 8 is a neuroinflammation inhibitor. Brain J Neurol 144:528–552. https://doi.org/10.1093/brain/awaa425
Kim WX, Ragonnaud E, Bodogai M, Illouz T, DeLuca M, McDevitt RA et al (2021) Therapeutic B-cell depletion reverses progression of Alzheimer’s disease. Nat Commun 12:2185. https://doi.org/10.1038/s41467-021-22479-4
Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J et al (2019) Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10:3887. https://doi.org/10.1038/s41467-019-11638-3
Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V et al (1950) LaFerla FM (2011) Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J Immunol Baltim Md 187:6539–6549. https://doi.org/10.4049/jimmunol.1100620
Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT et al (2021) Alzheimer disease Nat Rev Dis Primer 7:1–21. https://doi.org/10.1038/s41572-021-00269-y
Koper MJ, Van Schoor E, Ospitalieri S, Vandenberghe R, Vandenbulcke M, Arnim CAF et al (2020) Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol (Berl) 139:463–484. https://doi.org/10.1007/s00401-019-02103-y
Kosoy R, Fullard JF, Zeng B, Bendl J, Dong P, Rahman S et al (2022) Genetics of the human microglia regulome refines Alzheimer’s disease risk loci. Nat Genet 54:1145–1154. https://doi.org/10.1038/s41588-022-01149-1
Kovac A, Erickson MA, Banks WA (2011) Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation 8:139. https://doi.org/10.1186/1742-2094-8-139
Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U et al (2013) Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE 8:e60921. https://doi.org/10.1371/journal.pone.0060921
Kurz C, Walker L, Rauchmann B-S, Perneczky R (2022) Dysfunction of the blood–brain barrier in Alzheimer’s disease: Evidence from human studies. Neuropathol Appl Neurobiol 48:e12782. https://doi.org/10.1111/nan.12782
Kwon HS, Koh S-H (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9:42. https://doi.org/10.1186/s40035-020-00221-2
Lau S-F, Fu AKY, Ip NY (2023) Receptor–ligand interaction controls microglial chemotaxis and amelioration of Alzheimer’s disease pathology. J Neurochem 166:891–903. https://doi.org/10.1111/jnc.15933
Lau V, Ramer L, Tremblay M-È (2023) An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer’s disease. Nat Commun 14:1670. https://doi.org/10.1038/s41467-023-37304-3
LeBlanc AC, Xue R, Gambetti P (1996) Amyloid precursor protein metabolism in primary cell cultures of neurons, astrocytes, and microglia. J Neurochem 66:2300–2310. https://doi.org/10.1046/j.1471-4159.1996.66062300.x
Lee J-H, Kim J, Noh S, Lee H, Lee SY, Mun JY et al (2021) Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590:612–617. https://doi.org/10.1038/s41586-020-03060-3
Leng F, Hinz R, Gentleman S, Hampshire A, Dani M, Brooks DJ et al (2023) Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Mol Psychiatry 28:1303–1311. https://doi.org/10.1038/s41380-022-01878-z
Li Y, Xu P, Shan J, Sun W, Ji X, Chi T et al (2020) Interaction between hyperphosphorylated tau and pyroptosis in forskolin and streptozotocin induced AD models. Biomed Pharmacother 121:109618. https://doi.org/10.1016/j.biopha.2019.109618
Lian H, Litvinchuk A, Chiang AC-A, Aithmitti N, Jankowsky JL, Zheng H (2016) Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of alzheimer’s disease. J Neurosci 36:577–589. https://doi.org/10.1523/JNEUROSCI.2117-15.2016
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E et al (2013) Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 61:1134–1145. https://doi.org/10.1002/glia.22502
Lindberg C, Selenica M-LB, Westlind-Danielsson A, Schultzberg M (2005) Beta-amyloid protein structure determines the nature of cytokine release from rat microglia. J Mol Neurosci MN 27:1–12. https://doi.org/10.1385/JMN:27:1:001
Liu C, Cui G, Zhu M, Kang X, Guo H (2014) Neuroinflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol 7:8342–8355
Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B et al (1950) Fassbender K (2012) TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J Immunol Baltim Md 188:1098–1107. https://doi.org/10.4049/jimmunol.1101121
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H et al (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535:153–158. https://doi.org/10.1038/nature18629
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. https://doi.org/10.1038/nature14432
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR et al (2014) Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156:1193–1206. https://doi.org/10.1016/j.cell.2014.02.008
Lue L-F, Rydel R, Brigham EF, Yang L-B, Hampel H, Murphy GM Jr et al (2001) Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia 35:72–79. https://doi.org/10.1002/glia.1072
Mallach A, Zielonka M, van Lieshout V, An Y, Khoo JH, Vanheusden M et al (2024) Microglia-astrocyte crosstalk in the amyloid plaque niche of an Alzheimer’s disease mouse model, as revealed by spatial transcriptomics. Cell Rep. https://doi.org/10.1016/j.celrep.2024.114216
Mancuso R, Fattorelli N, Martinez-Muriana A, Davis E, Wolfs L, Van Den Daele J et al (2024) Xenografted human microglia display diverse transcriptomic states in response to Alzheimer’s disease-related amyloid-β pathology. Nat Neurosci 27:886–900. https://doi.org/10.1038/s41593-024-01600-y
Mancuso R, Fryatt G, Cleal M, Obst J, Pipi E, Monzón-Sandoval J et al (2019) CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain J Neurol 142:3243–3264. https://doi.org/10.1093/brain/awz241
Mangalmurti A, Lukens JR (2022) How neurons die in Alzheimer’s disease: Implications for neuroinflammation. Curr Opin Neurobiol 75:102575. https://doi.org/10.1016/j.conb.2022.102575
Mangiafico SP, Tuo Q-Z, Li X-L, Liu Y, Haralambous C, Ding X-L et al (2023) Tau suppresses microtubule-regulated pancreatic insulin secretion. Mol Psychiatry 28:3982–3993. https://doi.org/10.1038/s41380-023-02267-w
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M (2010) Alzheimer’s disease: clinical trials and drug development. Lancet Neurol 9:702–716. https://doi.org/10.1016/S1474-4422(10)70119-8
Maté de Gérando A, d’Orange M, Augustin E, Joséphine C, Aurégan G, Gaudin-Guérif M et al (2021) Neuronal tau species transfer to astrocytes and induce their loss according to tau aggregation state. Brain 144:1167–1182. https://doi.org/10.1093/brain/awab011
Matsumoto J, Takata F, Machida T, Takahashi H, Soejima Y, Funakoshi M et al (2014) Tumor necrosis factor-α-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation. Neurosci Lett 578:133–138. https://doi.org/10.1016/j.neulet.2014.06.052
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC et al (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330:1774. https://doi.org/10.1126/science.1197623
McAleese KE, Miah M, Graham S, Hadfield GM, Walker L, Johnson M et al (2021) Frontal white matter lesions in Alzheimer’s disease are associated with both small vessel disease and AD-associated cortical pathology. Acta Neuropathol (Berl) 142:937–950. https://doi.org/10.1007/s00401-021-02376-2
McAlpine CS, Park J, Griciuc A, Kim E, Choi SH, Iwamoto Y et al (2021) Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 595:701–706. https://doi.org/10.1038/s41586-021-03734-6
McCarthy JV, Twomey C, Wujek P (2009) Presenilin-dependent regulated intramembrane proteolysis and γ-secretase activity. Cell Mol Life Sci 66:1534–1555. https://doi.org/10.1007/s00018-009-8435-9
Medzhitov R (2010) Inflammation 2010: new adventures of an old flame. Cell 140:771–776. https://doi.org/10.1016/j.cell.2010.03.006
Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P (2009) Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 210:3–12. https://doi.org/10.1016/j.jneuroim.2009.02.003
Miners JS, Kehoe PG, Love S, Zetterberg H, Blennow K (2019) CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimers Res Ther 11:1–6. https://doi.org/10.1186/s13195-019-0534-8
Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R (2001) Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol (Berl) 101:249–255. https://doi.org/10.1007/s004010000284
Moonen S, Koper MJ, Van Schoor E, Schaeverbeke JM, Vandenberghe R, von Arnim CAF et al (2023) Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol (Berl) 145:175–195. https://doi.org/10.1007/s00401-022-02528-y
Mothes T, Portal B, Konstantinidis E, Eltom K, Libard S, Streubel-Gallasch L et al (2023) Astrocytic uptake of neuronal corpses promotes cell-to-cell spreading of tau pathology. Acta Neuropathol Commun 11:97. https://doi.org/10.1186/s40478-023-01589-8
Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP et al (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48:380-395.e6. https://doi.org/10.1016/j.immuni.2018.01.011
Mudher A, Colin M, Dujardin S, Medina M, Dewachter I, Alavi Naini SM et al (2017) What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol Commun 5:99. https://doi.org/10.1186/s40478-017-0488-7
Murray CA, Lynch MA (1998) Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci Off J Soc Neurosci 18:2974–2981. https://doi.org/10.1523/JNEUROSCI.18-08-02974.1998
Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang K-C, Wegiel J (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging 25:663–674. https://doi.org/10.1016/j.neurobiolaging.2004.01.007
Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F et al (2022) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health 7:e105–e125. https://doi.org/10.1016/S2468-2667(21)00249-8
Nicoll JAR, Weller RO (2003) A new role for astrocytes: beta-amyloid homeostasis and degradation. Trends Mol Med 9:281–282. https://doi.org/10.1016/s1471-4914(03)00109-6
Niewiadomska G, Niewiadomski W, Steczkowska M, Gasiorowska A (2021) Tau oligomers neurotoxicity. Life Basel Switz 11:28. https://doi.org/10.3390/life11010028
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318. https://doi.org/10.1126/science.1110647
Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z et al (2019) Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365:eaav9518. https://doi.org/10.1126/science.aav9518
Novikova G, Kapoor M, Tcw J, Abud EM, Efthymiou AG, Chen SX (2021) Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat Commun 12:1610. https://doi.org/10.1038/s41467-021-21823-y
Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H et al (2021) Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 20:e13296. https://doi.org/10.1111/acel.13296
Opland CK, Bryan MR, Harris B, McGillion-Moore J, Tian X, Chen Y et al (2023) Activity-dependent tau cleavage by caspase-3 promotes neuronal dysfunction and synaptotoxicity. iScience 26:106905. https://doi.org/10.1016/j.isci.2023.106905
Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I et al (2014) Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging 35:1–14. https://doi.org/10.1016/j.neurobiolaging.2013.07.008
Palacino JJ, Berechid BE, Alexander P, Eckman C, Younkin S, Nye JS et al (2000) Regulation of amyloid precursor protein processing by presenilin 1 (PS1) and PS2 in PS1 knockout cells. J Biol Chem 275:215–222. https://doi.org/10.1074/jbc.275.1.215
Pampuscenko K, Morkuniene R, Krasauskas L, Smirnovas V, Brown GC, Borutaite V (2023) Extracellular tau stimulates phagocytosis of living neurons by activated microglia via Toll-like 4 receptor–NLRP3 inflammasome–caspase-1 signalling axis. Sci Rep 13:10813. https://doi.org/10.1038/s41598-023-37887-3
Park G, Nhan HS, Tyan S-H, Kawakatsu Y, Zhang C, Navarro M et al (2020) Caspase activation and caspase-mediated cleavage of APP is associated with amyloid β-protein-induced synapse loss in alzheimer’s disease. Cell Rep 31:107839. https://doi.org/10.1016/j.celrep.2020.107839
Perea JR, López E, Díez-Ballesteros JC, Ávila J, Hernández F, Bolós M (2019) Extracellular monomeric tau is internalized by astrocytes. Front Neurosci 13:442. https://doi.org/10.3389/fnins.2019.00442
Perez-Nievas BG, Serrano-Pozo A (2018) Deciphering the astrocyte reaction in alzheimer’s disease. Front Aging Neurosci 10:114. https://doi.org/10.3389/fnagi.2018.00114
Pham C, Hérault K, Oheim M, Maldera S, Vialou V, Cauli B et al (2021) Astrocytes respond to a neurotoxic Aβ fragment with state-dependent Ca2+ alteration and multiphasic transmitter release. Acta Neuropathol Commun 9:44. https://doi.org/10.1186/s40478-021-01146-1
Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W et al (2013) Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med 5:77. https://doi.org/10.1126/scitranslmed.3005615
Prater KE, Green KJ, Mamde S, Sun W, Cochoit A, Smith CL et al (2023) Human microglia show unique transcriptional changes in Alzheimer’s disease. Nat Aging 3:894–907. https://doi.org/10.1038/s43587-023-00424-y
Prinz M, Masuda T, Wheeler MA, Quintana FJ (2021) Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu Rev Immunol 39:251–277. https://doi.org/10.1146/annurev-immunol-093019-110159
Qian Z, Qin J, Lai Y, Zhang C, Zhang X (2023) Large-scale integration of single-cell RNA-Seq data reveals astrocyte diversity and transcriptomic modules across six central nervous system disorders. Biomolecules 13:692. https://doi.org/10.3390/biom13040692
Rajani RM, Ellingford R, Hellmuth M, Harris SS, Taso OS, Graykowski D et al (2024) Selective suppression of oligodendrocyte-derived amyloid beta rescues neuronal dysfunction in Alzheimer’s Disease. PLoS Biol. https://doi.org/10.1101/2024.06.21.600003
Ravichandran KA, Heneka MT (2024) Inflammasomes in neurological disorders—mechanisms and therapeutic potential. Nat Rev Neurol 20:67–83. https://doi.org/10.1038/s41582-023-00915-x
Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K et al (2017) The lifespan and turnover of microglia in the human brain. Cell Rep 20:779–784. https://doi.org/10.1016/j.celrep.2017.07.004
Richetin K, Steullet P, Pachoud M, Perbet R, Parietti E, Maheswaran M et al (2020) Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat Neurosci 23:1567–1579. https://doi.org/10.1038/s41593-020-00728-x
Ringman JM, O’Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y et al (2007) Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer’s disease mutations. Brain 130:1767–1776. https://doi.org/10.1093/brain/awm102
Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A (2009) Astroglia in dementia and Alzheimer’s disease. Cell Death Differ 16:378–385. https://doi.org/10.1038/cdd.2008.172
Rodríguez JJ, Zallo F, Gardenal E, Cabot J, Busquets X (2023) Prominent and conspicuous astrocyte atrophy in human sporadic and familial Alzheimer’s disease. Brain Struct Funct 228:2103–2113. https://doi.org/10.1007/s00429-023-02707-x
Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 323:170–182. https://doi.org/10.1016/j.neuroscience.2015.01.007
Rong Y, Ji C, Wang Z, Ge X, Wang J, Ye W et al (2021) Small extracellular vesicles encapsulating CCL2 from activated astrocytes induce microglial activation and neuronal apoptosis after traumatic spinal cord injury. J Neuroinflammation 18:196. https://doi.org/10.1186/s12974-021-02268-y
Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark GT, Ingelsson M et al (2017) Human Astrocytes Transfer Aggregated Alpha-Synuclein via Tunneling Nanotubes. J Neurosci Off J Soc Neurosci 37:11835–11853. https://doi.org/10.1523/JNEUROSCI.0983-17.2017
Rostami J, Mothes T, Kolahdouzan M, Eriksson O, Moslem M, Bergström J et al (2021) Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J Neuroinflammation 18:124. https://doi.org/10.1186/s12974-021-02158-3
Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao C-C, Ardura-Fabregat A et al (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557:724–728. https://doi.org/10.1038/s41586-018-0119-x
Rustenhoven J, Jansson D, Smyth LC, Dragunow M (2017) Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol Sci 38:291–304. https://doi.org/10.1016/j.tips.2016.12.001
Sadick JS, O’Dea MR, Hasel P, Dykstra T, Faustin A, Liddelow SA (2022) Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110:1788-1805.e10. https://doi.org/10.1016/j.neuron.2022.03.008
Saha P, Guha S, Biswas SC (2020) P38K and JNK pathways are induced by amyloid-β in astrocyte: implication of MAPK pathways in astrogliosis in Alzheimer’s disease. Mol Cell Neurosci 108:103551. https://doi.org/10.1016/j.mcn.2020.103551
Salvadores N, Moreno-Gonzalez I, Gamez N, Quiroz G, Vegas-Gomez L, Escandón M et al (2022) Aβ oligomers trigger necroptosis-mediated neurodegeneration via microglia activation in Alzheimer’s disease. Acta Neuropathol Commun 10:31. https://doi.org/10.1186/s40478-022-01332-9
Sasmita AO, Depp C, Nazarenko T, Sun T, Siems SB, Yu X et al (2023) Oligodendrocytes and neurons contribute to amyloid-β deposition in Alzheimer’s disease. bioRxiv. https://doi.org/10.1101/2023.12.11.570514
Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ (2002) Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia aging study. Ann Neurol 52:168–174. https://doi.org/10.1002/ana.10265
Schultz N, Nielsen HM, Minthon L, Wennström M (2014) Involvement of matrix metalloproteinase-9 in amyloid-β 1–42–induced shedding of the pericyte proteoglycan NG2. J Neuropathol Exp Neurol 73:684–692. https://doi.org/10.1097/NEN.0000000000000084
Sebastian Monasor L, Müller SA, Colombo AV, Tanrioever G, König J, Roth S et al (2020) Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models. Elife 9:54083. https://doi.org/10.7554/eLife.54083
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. https://doi.org/10.15252/emmm.201606210
Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013) Deficiency in mural vascular cells coincides with blood-brain barrier disruption in alzheimer’s disease. Brain Pathol 23:303–310. https://doi.org/10.1111/bpa.12004
Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB et al (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating the intracellular nucleation from soluble to particulate ligands in sterile inflammation. Nat Immunol 14:812–820. https://doi.org/10.1038/ni.2639
Shen H, Han C, Yang Y, Guo L, Sheng Y, Wang J et al (2021) Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer’s disease. Brain Behav 11:e02063. https://doi.org/10.1002/brb3.2063
Shi H, Koronyo Y, Rentsendorj A, Regis GC, Sheyn J, Fuchs D-T et al (2020) Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol (Berl) 139:813–836. https://doi.org/10.1007/s00401-020-02134-w
Shirai Y (1921) On the transplantation of the rat sarcoma in adult heterogenous animals. Jap Med World 1:14–15
Sidoryk-Wegrzynowicz M, Gerber YN, Ries M, Sastre M, Tolkovsky AM, Spillantini MG (2017) Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions. Acta Neuropathol Commun 5:89. https://doi.org/10.1186/s40478-017-0478-9
Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K (2007) Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55:412–424. https://doi.org/10.1002/glia.20468
Simard AR, Soulet D, Gowing G, Julien J-P, Rivest S (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in alzheimer’s disease. Neuron 49:489–502. https://doi.org/10.1016/j.neuron.2006.01.022
Skaper SD, Evans NA, Soden PE, Rosin C, Facci L, Richardson JC (2009) Oligodendrocytes are a novel source of amyloid peptide generation. Neurochem Res 34:2243–2250. https://doi.org/10.1007/s11064-009-0022-9
Smith AM, Davey K, Tsartsalis S, Khozoie C, Fancy N, Tang SS et al (2022) Diverse human astrocyte and microglial transcriptional responses to Alzheimer’s pathology. Acta Neuropathol (Berl) 143:75–91. https://doi.org/10.1007/s00401-021-02372-6
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. https://doi.org/10.1016/j.tins.2009.08.002
Sofroniew MV (2014) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20:160–172. https://doi.org/10.1177/1073858413504466
Sosna J, Philipp S, Albay R, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM et al (2018) Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener 13:11. https://doi.org/10.1186/s13024-018-0244-x
Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA et al (2019) Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat Commun 10:3758. https://doi.org/10.1038/s41467-019-11674-z
Spanos F, Liddelow SA (2020) An overview of astrocyte responses in genetically induced alzheimer’s disease mouse models. Cells 9:2415. https://doi.org/10.3390/cells9112415
Stadelmann C, Bruck W, Bancher C, Jellinger K, Lassmann H (1998) Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol 57:456–464. https://doi.org/10.1097/00005072-199805000-00009
Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K et al (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in alzheimer’s disease. Am J Pathol 155:1459–1466
Stancu IC, Lodder C, Botella Lucena P, Vanherle S, Gutiérrez de Ravé M, Terwel D et al (2022) The NLRP3 inflammasome modulates tau pathology and neurodegeneration in a tauopathy model. Glia 70:1117–1132. https://doi.org/10.1002/glia.24160
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A et al (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11:155–161. https://doi.org/10.1038/ni.1836
Sudwarts A, Thinakaran G (2023) Alzheimer’s genes in microglia: a risk worth investigating. Mol Neurodegener 18:90. https://doi.org/10.1186/s13024-023-00679-4
Sun N, Victor MB, Park YP, Xiong X, Scannail AN, Leary N et al (2023) Human microglial state dynamics in Alzheimer’s disease progression. Cell 186:4386-4403.e29. https://doi.org/10.1016/j.cell.2023.08.037
Taipa R, Ferreira V, Brochado P, Robinson A, Reis I, Marques F et al (2018) Inflammatory pathology markers (activated microglia and reactive astrocytes) in early and late onset Alzheimer disease: a post mortem study. Neuropathol Appl Neurobiol 44:298–313. https://doi.org/10.1111/nan.12445
Takahashi H, Klein ZA, Bhagat SM, Kaufman AC, Kostylev MA, Ikezu T et al (2017) Opposing effects of progranulin deficiency on amyloid and tau pathologies via microglial TYROBP network. Acta Neuropathol (Berl) 133:785–807. https://doi.org/10.1007/s00401-017-1668-z
Tan M-S, Tan L, Jiang T, Zhu X-C, Wang H-F, Jia C-D et al (2014) Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis 5:e1382. https://doi.org/10.1038/cddis.2014.348
Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB et al (2007) Inflammatory markers and the risk of Alzheimer disease. Neurology 68:1902–1908. https://doi.org/10.1212/01.wnl.0000263217.36439.da
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E et al (2015) Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol 11:457–470. https://doi.org/10.1038/nrneurol.2015.119
Taylor X, Cisternas P, You Y, You Y, Xiang S, Marambio Y et al (2020) A1 reactive astrocytes and a loss of TREM2 are associated with an early stage of pathology in a mouse model of cerebral amyloid angiopathy. J Neuroinflammation 17:223. https://doi.org/10.1186/s12974-020-01900-7
Taylor X, Clark IM, Fitzgerald GJ, Oluoch H, Hole JT, DeMattos RB et al (2023) Amyloid-β (Aβ) immunotherapy induced microhemorrhages are associated with activated perivascular macrophages and peripheral monocyte recruitment in Alzheimer’s disease mice. Mol Neurodegener 18:1–16. https://doi.org/10.1186/s13024-023-00649-w
Thal DR, Gawor K, Moonen S (2024) Regulated cell death and its role in Alzheimer’s disease and amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 147:69. https://doi.org/10.1007/s00401-024-02722-0
Tzioras M, Daniels MJD, Davies C, Baxter P, King D, McKay S et al (2023) Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer’s disease via MFG-E8. Cell Rep Med 4:101175. https://doi.org/10.1016/j.xcrm.2023.101175
Uekawa K, Hattori Y, Ahn SJ, Seo J, Casey N, Anfray A et al (2023) Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress. Mol Neurodegener 18:1–20. https://doi.org/10.1186/s13024-023-00660-1
Ungerleider K, Beck J, Lissa D, Turnquist C, Horikawa I, Harris BT et al (2021) Astrocyte senescence and SASP in neurodegeneration: tau joins the loop. Cell Cycle 20:752–764. https://doi.org/10.1080/15384101.2021.1909260
United Nations (2022) World population prospects Highlights, 2019 revision Highlights, 2019 revision
Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S et al (2019) A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22:1021–1035. https://doi.org/10.1038/s41593-019-0393-4
Vaquer-Alicea J, Diamond MI (2019) Propagation of protein aggregation in neurodegenerative diseases. Annu Rev Biochem 88:785–810. https://doi.org/10.1146/annurev-biochem-061516-045049
Vasile F, Dossi E, Rouach N (2017) Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct 222:2017–2029. https://doi.org/10.1007/s00429-017-1383-5
Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D et al (2017) Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552:355–361. https://doi.org/10.1038/nature25158
Verdile G, Gandy SE, Martins RN (2007) The Role of Presenilin and its Interacting Proteins in the Biogenesis of Alzheimer’s Beta Amyloid. Neurochem Res 32:609–623. https://doi.org/10.1007/s11064-006-9131-x
Verkhratsky A, Butt A, Li B, Illes P, Zorec R, Semyanov A et al (2023) Astrocytes in human central nervous system diseases: a frontier for new therapies. Signal Transduct Target Ther 8:1–37. https://doi.org/10.1038/s41392-023-01628-9
Verkhratsky A, Zorec R, Parpura V (2017) Stratification of astrocytes in healthy and diseased brain. Brain Pathol Zurich Switz 27:629–644. https://doi.org/10.1111/bpa.12537
Viejo L, Noori A, Merrill E, Das S, Hyman BT, Serrano-Pozo A (2022) Systematic review of human post-mortem immunohistochemical studies and bioinformatics analyses unveil the complexity of astrocyte reaction in Alzheimer’s disease. Neuropathol Appl Neurobiol 48:e12753. https://doi.org/10.1111/nan.12753
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LBJ, Tiwari-Woodruff S et al (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522. https://doi.org/10.1523/JNEUROSCI.1514-09.2009
Wachter A, Woodbury ME, Lombardo S, Abdourahman A, Wuest C, McGlame E et al (2024) Landscape of brain myeloid cell transcriptome along the spatiotemporal progression of Alzheimer’s disease reveals distinct sequential responses to Aβ and tau. Acta Neuropathol (Berl) 147:1–20. https://doi.org/10.1007/s00401-024-02704-2
Walker DG, Link J, Lue L-F, Dalsing-Hernandez JE, Boyes BE (2006) Gene expression changes by amyloid beta peptide-stimulated human postmortem brain microglia identify activation of multiple inflammatory processes. J Leukoc Biol 79:596–610. https://doi.org/10.1189/jlb.0705377
Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W et al (2007) Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 20:947–956. https://doi.org/10.1159/000110455
Wang C, Fan L, Khawaja RR, Liu B, Zhan L, Kodama L et al (2022) Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat Commun 13:1969. https://doi.org/10.1038/s41467-022-29552-6
Wang H, Luo W, Zhang Y, Li Y-M, Thinakaran G, Greengard P et al (2004) Presenilins and γ-secretase inhibitors affect intracellular trafficking and cell surface localization of the γ-secretase complex components*. J Biol Chem 279:40560–40566. https://doi.org/10.1074/jbc.M404345200
Wang L, Nykänen N-P, Western D, Gorijala P, Timsina J, Li F et al (2024) Proteo-genomics of soluble TREM2 in cerebrospinal fluid provides novel insights and identifies novel modulators for Alzheimer’s disease. Mol Neurodegener 19:1. https://doi.org/10.1186/s13024-023-00687-4
Wang P, Ye Y (2021) Filamentous recombinant human Tau activates primary astrocytes via an integrin receptor complex. Nat Commun 12:95. https://doi.org/10.1038/s41467-020-20322-w
Wang T, Perera ND, Chiam MDF, Cuic B, Wanniarachchillage N, Tomas D et al (2020) Necroptosis is dispensable for motor neuron degeneration in a mouse model of ALS. Cell Death Differ 27:1728–1739. https://doi.org/10.1038/s41418-019-0457-8
Wang X, Deckert M, Xuan NT, Nishanth G, Just S, Waisman A et al (2013) Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-κB- and STAT1-dependent chemokine production in astrocytes. Acta Neuropathol (Berl) 126:711–724. https://doi.org/10.1007/s00401-013-1183-9
Weiskopf K, Schnorr PJ, Pang WW, Chao MP, Chhabra A, Seita J et al (2017) Myeloid cell origins, differentiation, and clinical implications. Myeloid cells in health and disease. Wiley, pp 857–875
Wendt S, Maricos M, Vana N, Meyer N, Guneykaya D, Semtner M et al (2017) Changes in phagocytosis and potassium channel activity in microglia of 5xFAD mice indicate alterations in purinergic signaling in a mouse model of Alzheimer’s disease. Neurobiol Aging 58:41–53. https://doi.org/10.1016/j.neurobiolaging.2017.05.027
Wheeler MA, Clark IC, Lee H-G, Li Z, Linnerbauer M, Rone JM et al (2023) Droplet-based forward genetic screening of astrocyte–microglia cross-talk. Science 379:1023–1030. https://doi.org/10.1126/science.abq4822
Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I (2023) Hallmarks of neurodegenerative diseases. Cell 186:693–714. https://doi.org/10.1016/j.cell.2022.12.032
Wilson EN, Wang C, Swarovski MS, Zera KA, Ennerfelt HE, Wang Q et al (2024) TREM1 disrupts myeloid bioenergetics and cognitive function in aging and Alzheimer disease mouse models. Nat Neurosci. https://doi.org/10.1038/s41593-024-01610-w
Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S et al (2019) Complement C3 is activated in human ad brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep 28:2111-2123.e6. https://doi.org/10.1016/j.celrep.2019.07.060
Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP et al (2001) Amyloid-β peptides are cytotoxic to oligodendrocytes. J Neurosci 21:RC118
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH et al (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11:251–253. https://doi.org/10.1038/nn2047
Yang F, Zhao K, Zhang X, Zhang J, Xu B (2016) ATP induces disruption of tight junction proteins via IL-1 beta-dependent MMP-9 activation of human blood-brain barrier in vitro. Neural Plast 2016:8928530. https://doi.org/10.1155/2016/8928530
Yang Y, Andersson P, Hosaka K, Zhang Y, Cao R, Iwamoto H et al (2016) The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nat Commun 7:11385. https://doi.org/10.1038/ncomms11385
Yap JKY, Pickard BS, Chan EWL, Gan SY (2019) The role of neuronal NLRP1 inflammasome in alzheimer’s disease: bringing neurons into the neuroinflammation game. Mol Neurobiol 56:7741–7753. https://doi.org/10.1007/s12035-019-1638-7
Ye H, Han Y, Li P, Su Z, Huang Y (2022) The role of post-translational modifications on the structure and function of tau protein. J Mol Neurosci 72:1557–1571. https://doi.org/10.1007/s12031-022-02002-0
Yoshiyama Y, Higuchi M, Zhang B, Huang S-M, Iwata N, Saido TC et al (2007) Synapse loss and microglial activation precede tangles in a P301s tauopathy mouse model. Neuron 53:337–351. https://doi.org/10.1016/j.neuron.2007.01.010
Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X (2021) Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther 6:1–21. https://doi.org/10.1038/s41392-021-00507-5
Zhang B, Gaiteri C, Bodea L-G, Wang Z, McElwee J, Podtelezhnikov AA et al (2013) Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153:707–720. https://doi.org/10.1016/j.cell.2013.03.030
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S et al (2019) Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22:719–728. https://doi.org/10.1038/s41593-019-0372-9
Zhang X, Wang R, Hu D, Sun X, Fujioka H, Chan E et al (2020) Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer’s disease. Sci Adv. https://doi.org/10.1126/sciadv.abb8680
Zhao J, O’Connor T, Vassar R (2011) The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation 8:150. https://doi.org/10.1186/1742-2094-8-150
Zhao J, Paganini L, Mucke L, Gordon M, Refolo L, Carman M et al (1996) β-secretase processing of the β-amyloid precursor protein in transgenic mice is efficient in neurons but inefficient in astrocytes *. J Biol Chem 271:31407–31411. https://doi.org/10.1074/jbc.271.49.31407
Zhao J, Wu H, Tang X (2021) Tau internalization: a complex step in tau propagation. Ageing Res Rev 67:101272. https://doi.org/10.1016/j.arr.2021.101272
Zhou Y, Song WM, Andhey PS, Swain A, Levy T, Miller KR et al (2020) Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med 26:131–142. https://doi.org/10.1038/s41591-019-0695-9
Zhu K, Liang W, Ma Z, Xu D, Cao S, Lu X et al (2018) Necroptosis promotes cell-autonomous activation of proinflammatory cytokine gene expression. Cell Death Dis 9:1–16. https://doi.org/10.1038/s41419-018-0524-y
Zindel J, Kubes P (2020) DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol 15:493–518. https://doi.org/10.1146/annurev-pathmechdis-012419-032847
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Botella Lucena, P., Heneka, M.T. Inflammatory aspects of Alzheimer’s disease. Acta Neuropathol 148, 31 (2024). https://doi.org/10.1007/s00401-024-02790-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00401-024-02790-2