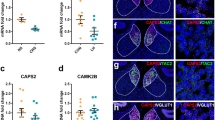
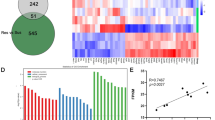
Abstract
Since the discovery of ketamine anti-depressant effects in last decade, it has effectively revitalized interest in investigating excitatory synapses hypothesis in the pathogenesis of depression. In the present study, we aimed to reveal the excitatory synaptic regulation of corticotropin-releasing hormone (CRH) neuron in the hypothalamus, which is the driving force in hypothalamic–pituitary–adrenal (HPA) axis regulation. This study constitutes the first observation of an increased density of PSD-93-CRH co-localized neurons in the hypothalamic paraventricular nucleus (PVN) of patients with major depression. PSD-93 overexpression in CRH neurons in the PVN induced depression-like behaviors in mice, accompanied by increased serum corticosterone level. PSD-93 knockdown relieved the depression-like phenotypes in a lipopolysaccharide (LPS)-induced depression model. Electrophysiological data showed that PSD-93 overexpression increased CRH neurons synaptic activity, while PSD-93 knockdown decreased CRH neurons synaptic activity. Furthermore, we found that LPS induced increased the release of glutamate from microglia to CRH neurons resulted in depression-like behaviors using fiber photometry recordings. Together, these results show that PSD-93 is involved in the pathogenesis of depression via increasing the synaptic activity of CRH neurons in the PVN, leading to the hyperactivity of the HPA axis that underlies depression-like behaviors.






Similar content being viewed by others
References
Amionff M, Boller F, Swaab D (2003) Handbook of clinical neurology, Vol. 79. The human hypothalamus: basic and clinical aspects Part I. Elsevier, Amsterdam
Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB (1987) CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry 144:873–877. https://doi.org/10.1176/ajp.144.7.873
Bao AM, Swaab DF (2019) The human hypothalamus in mood disorders: the HPA axis in the center. IBRO Rep 6:45–53. https://doi.org/10.1016/j.ibror.2018.11.008
Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN (2005) Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain 128:1301–1313. https://doi.org/10.1093/brain/awh448
Barger SW, Basile AS (2001) Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem 76:846–854
Barger SW, Goodwin ME, Porter MM, Beggs ML (2007) Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem 101:1205–1213. https://doi.org/10.1111/j.1471-4159.2007.04487.x
Beliveau BJ, Kishi JY, Nir G, Sasaki HM, Saka SK, Nguyen SC et al (2018) OligoMiner provides a rapid, flexible environment for the design of genome-scale oligonucleotide in situ hybridization probes. Proc Natl Acad Sci 115:E2183–E2192
Caceres A, Binder L, Payne M, Bender P, Rebhun L, Steward O (1984) Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci 4:394–410
Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 124:307–321. https://doi.org/10.3109/00207454.2013.833510
Carlisle HJ, Fink AE, Grant SG, O’Dell TJ (2008) Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol 586:5885–5900. https://doi.org/10.1113/jphysiol.2008.163469
Cai L, Yan XB, Chen XN, Meng QY, Zhou JN (2010) Chronic all-trans retinoic acid administration induced hyperactivity of HPA axis and behavioral changes in young rats. European Neuropsychopharmacol 20(12):839–847. https://doi.org/10.1016/j.euroneuro.2010.06.019
Chen X-N, Meng Q-Y, Bao A-M, Swaab DF, Wang G-H, Zhou J-N (2009) The involvement of retinoic acid receptor-α in corticotropin-releasing hormone gene expression and affective disorders. Biol Psychiat 66:832–839. https://doi.org/10.1016/j.biopsych.2009.05.031
Chen P, Lou S, Huang ZH, Wang Z, Shan QH, Wang Y et al (2020) Prefrontal cortex corticotropin-releasing factor neurons control behavioral style selection under challenging situations. Neuron 106(301–315):e307. https://doi.org/10.1016/j.neuron.2020.01.033
Choi HMT, Schwarzkopf M, Fornace ME, Acharya A, Artavanis G, Stegmaier J et al (2018) Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development. https://doi.org/10.1242/dev.165753
Cserep C, Posfai B, Denes A (2021) Shaping neuronal fate: functional heterogeneity of direct microglia-neuron interactions. Neuron 109:222–240. https://doi.org/10.1016/j.neuron.2020.11.007
Duman RS, Aghajanian GK, Sanacora G, Krysta JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. https://doi.org/10.1038/nm.4050
Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC et al (2013) Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16:69–82. https://doi.org/10.1017/S1461145712000016
Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA (2006) Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron 52:307–320. https://doi.org/10.1016/j.neuron.2006.09.012
Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J et al (2007) Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32:516–531. https://doi.org/10.1016/j.psyneuen.2007.03.005
Fuchs E, Czeh B, Flügge G (2004) Examining novel concepts of the pathophysiology of depression in the chronic psychosocial stress paradigm in tree shrews. Behav Pharmacol 15:315–325
Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y (2007) Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci 10:186–195. https://doi.org/10.1038/nn1837
Gao TH, Ni RJ, Liu S, Tian Y, Wei J, Zhao L et al (2021) Chronic lithium exposure attenuates ketamine-induced mania-like behavior and c-Fos expression in the forebrain of mice. Pharmacol Biochem Behav 202:173108. https://doi.org/10.1016/j.pbb.2021.173108
Goh JJL, Chou N, Seow WY, Ha N, Cheng CPP, Chang YC et al (2020) Highly specific multiplexed RNA imaging in tissues with split-FISH. Nat Methods 17:689–693. https://doi.org/10.1038/s41592-020-0858-0
Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A et al (2014) VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci 17:1751–1758. https://doi.org/10.1038/nn.3872
Hart BL (1988) Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137
Hashimoto K (2019) Rapid-acting anti-depressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci 73:613–627. https://doi.org/10.1111/pcn.12902
Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G et al (2018) Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways (2018). Nat Commun. https://doi.org/10.1038/s41467-018-05310-5
Hu P, Liu J, Zhao J, Qi XR, Qi CC, Lucassen PJ et al (2013) All-trans retinoic acid-induced hypothalamus-pituitary-adrenal hyperactivity involves glucocorticoid receptor dysregulation. Transl Psychiatry 3:e336. https://doi.org/10.1038/tp.2013.98
Keck ME, Holsboer F (2001) Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides 22:835–844. https://doi.org/10.1016/S0196-9781(01)00398-9
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr et al (2017) HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 22:527–536. https://doi.org/10.1038/mp.2016.120
Kim E, Cho KO, Rothschild A, Sheng M (1996) Heteromultimerization and NMDA receptor-clustering activity of Chapsyn-110, a member of the PSD-95 family of proteins. Neuron 17:103–113
Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the post-synaptic density protein PSD-95. Science 269:1737–1740
Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB et al (2010) Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci 30:703–713. https://doi.org/10.1523/JNEUROSCI.4214-09.2010
Kruger JM, Favaro PD, Liu M, Kitlinska A, Huang X, Raabe M et al (2013) Differential roles of postsynaptic density-93 isoforms in regulating synaptic transmission. J Neurosci 33:15504–15517. https://doi.org/10.1523/JNEUROSCI.0019-12.2013
Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS (2019) Ketamine: a paradigm shift for depression research and treatment. Neuron 101:774–778. https://doi.org/10.1016/j.neuron.2019.02.005
Levy BH, Tasker JG (2012) Synaptic regulation of the hypothalamic–pituitary–adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neurosci. https://doi.org/10.3389/fncel.2012.00024
Lin H-C, Wan F-J, Kang B-H, Wu C-C, Tseng C-J (1999) Systemic administration of lipopolysaccharide induces release of nitric oxide and glutamate and c-fos expression in the nucleus tractus solitarii of rats. Hypertension 33:1218–1224
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mai J, Assheuer J, Paxinos G (2004) Atlas of the human brain. Elsevier Academic Press, Amsterdam
Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Muller JA et al (2018) Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat Methods 15:936–939. https://doi.org/10.1038/s41592-018-0171-3
Mayer SE, Lopez-Duran NL, Sen S, Abelson JL (2018) Chronic stress, hair cortisol and depression: a prospective and longitudinal study of medical internship. Psychoneuroendocrinology 92:57–65. https://doi.org/10.1016/j.psyneuen.2018.03.020
McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH (2007) Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res 1127:108–118. https://doi.org/10.1016/j.brainres.2006.09.011
McGovern DJ, Polter AM, Root DH (2021) Neurochemical signaling of reward and aversion to ventral tegmental area glutamate neurons. J Neurosci 41:5471–5486. https://doi.org/10.1523/JNEUROSCI.1419-20.2021
Mello BS, Monte AS, McIntyre RS, Soczynska JK, Custodio CS, Cordeiro RC et al (2013) Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res 47:1521–1529. https://doi.org/10.1016/j.jpsychires.2013.06.008
Meng FT, Ni RJ, Zhang Z, Zhao J, Liu YJ, Zhou JN (2011) Inhibition of oestrogen biosynthesis induces mild anxiety in C57BL/6J ovariectomized female mice. Neurosci Bull 27:241–250. https://doi.org/10.1007/s12264-011-1014-8
Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN et al (2019) Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. https://doi.org/10.1126/science.aat8078
Murugan M, Ling E-A, Kaur C (2013) Glutamate and microglia. CNS Neurol Disord Drug Targets (Former Curr Drug Targets CNS Neurol Disord) 12:773–784
Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K et al (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344. https://doi.org/10.1126/science.6334362
Ni RJ, Shu YM, Wang J, Yin JC, Xu L, Zhou JN (2014) Distribution of vasopressin, oxytocin and vasoactive intestinal polypeptide in the hypothalamus and extrahypothalamic regions of tree shrews. Neuroscience 265:124–136. https://doi.org/10.1016/j.neuroscience.2014.01.034
Niethammer M, Kim E, Sheng M (1996) Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci 16:2157–2163
Nishino S, Mignot E, Benson KL, Zarcone VP Jr (1998) Cerebrospinal fluid prostaglandins and corticotropin releasing factor in schizophrenics and controls: relationship to sleep architecture. Psychiatry Res 78:141–150. https://doi.org/10.1016/s0165-1781(98)00012-2
O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N et al (2009) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14:511–522. https://doi.org/10.1038/sj.mp.4002148
Ohgi Y, Futamura T, Kikuchi T, Hashimoto K (2013) Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 103:853–859. https://doi.org/10.1016/j.pbb.2012.12.003
Painsipp E, Kofer MJ, Sinner F, Holzer P (2011) Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS ONE 6:e20719. https://doi.org/10.1371/journal.pone.0020719
Pariante CM, Lightman SL (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468. https://doi.org/10.1016/j.tins.2008.06.006
Park YG, Sohn CH, Chen R, McCue M, Yun DH, Drummond GT et al (2018) Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat Biotechnol. https://doi.org/10.1038/nbt.4281
Patrizio M, Levi G (1994) Glutamate production by cultured microglia: differences between rat and mouse, enhancement by lipopolysaccharide and lack effect of HIV coat protein gp120 and depolarizing agents. Neurosci Lett 178:184–189. https://doi.org/10.1016/0304-3940(94)90755-2
Peng WH, Lo KL, Lee YH, Hung TH, Lin YC (2007) Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci 81:933–938. https://doi.org/10.1016/j.lfs.2007.08.003
Piani D, Spranger M, Frei K, Schaffner A, Fontana A (1992) Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol 22:2429–2436
Porsolt RD, Bertin A, Jalfre M (1978) Behavioural despair in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51:291–294
Qi CC, Zhang Z, Fang H, Liu J, Zhou N, Ge JF et al (2014) Antidepressant effects of abscisic acid mediated by the downregulation of corticotrophin-releasing hormone gene expression in rats. Int J Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyu006
Qin XY, Fang H, Shan QH, Qi CC, Zhou JN (2020) All-trans retinoic acid-induced abnormal hippocampal expression of synaptic genes SynDIG1 and DLG2 is correlated with anxiety or depression-like behavior in mice. Int J Mol Sci. https://doi.org/10.3390/ijms21082677
Raadsheer FC, Hoogendijk WJG, Stam FC, Tilders FJH, Swaab DF (1994) Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed-patients. Neuroendocrinology 60:436–444. https://doi.org/10.1159/000126778
Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF (1995) Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry 152:1372–1376. https://doi.org/10.1176/ajp.152.9.1372
Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis M (2007) Hypothalamus–pituitary–adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience 146:1734–1742. https://doi.org/10.1016/j.neuroscience.2007.03.027
Schindler S, Schmidt L, Stroske M, Storch M, Anwander A, Trampel R et al (2019) Hypothalamus enlargement in mood disorders. Acta Psychiatr Scand 139:56–67. https://doi.org/10.1111/acps.12958
Schnell SA, Staines WA, Wessendorf MW (1999) Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 47:719–730. https://doi.org/10.1177/002215549904700601
Shen Y, Connor TJ, Nolan Y, Kelly JP, Leonard BE (1999) Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci 65:1773–1786. https://doi.org/10.1016/s0024-3205(99)00430-0
Shao W, Zhang S, Tang M, Zhang X, Zhou Z, Yin Y et al (2013) Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin. Nature 494(7435):90–94. https://doi.org/10.1038/nature11748
Siew LK, Love S, Dawbarn D, Wilcock GK, Allen SJ (2004) Measurement of pre- and post-synaptic proteins in cerebral cortex: effects of post-mortem delay. J Neurosci Methods 139:153–159. https://doi.org/10.1016/j.jneumeth.2004.04.020
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461. https://doi.org/10.1038/nature10287
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Stokes PE (1995) The potential role of excessive cortisol induced by HPA hyperfunction in the pathogenesis of depression. Eur Neuropsychopharmacol 5(Suppl):77–82. https://doi.org/10.1016/0924-977x(95)00039-r
Stokes PE, Sikes CR (1991) Hypothalamic-pituitary-adrenal axis in psychiatric disorders. Annu Rev Med 42:519–531. https://doi.org/10.1146/annurev.me.42.020191.002511
Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P (2004) Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29:2007–2017. https://doi.org/10.1038/sj.npp.1300532
Sulakhiya K, Kumar P, Jangra A, Dwivedi S, Hazarika NK, Baruah CC et al (2014) Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur J Pharmacol 744:124–131. https://doi.org/10.1016/j.ejphar.2014.09.049
Sun Q, Turrigiano GG (2011) PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J Neurosci 31:6800–6808. https://doi.org/10.1523/JNEUROSCI.5616-10.2011
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R et al (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281:21362–21368. https://doi.org/10.1074/jbc.M600504200
Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X (2015) An excitatory synapse hypothesis of depression. Trends Neurosci 38:279–294. https://doi.org/10.1016/j.tins.2015.03.003
Trabzuni D, Ramasamy A, Imran S, Walker R, Smith C, Weale ME et al (2013) Widespread sex differences in gene expression and splicing in the adult human brain. Nat Commun 4:2771. https://doi.org/10.1038/ncomms3771
Tsai SF, Liu YW, Kuo YM (2019) Acute and long-term treadmill running differentially induce c-Fos expression in region- and time-dependent manners in mouse brain. Brain Struct Funct 224:2677–2689. https://doi.org/10.1007/s00429-019-01926-5
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B et al (2013) NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38:1609–1616. https://doi.org/10.1038/npp.2013.71
Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF (2008) Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry 13(786–799):741. https://doi.org/10.1038/mp.2008.38
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S et al (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554:317
Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M et al (2001) Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology 24:531–544. https://doi.org/10.1016/S0893-133X(00)00226-8
Yu X, Jiang X, Zhang X, Chen Z, Xu L, Chen L et al (2016) The effects of fisetin on lipopolysaccharide-induced depressive-like behavior in mice. Metab Brain Dis 31:1011–1021. https://doi.org/10.1007/s11011-016-9839-5
Zhang M, Xu JT, Zhu X, Wang Z, Zhao X, Hua Z et al (2010) Postsynaptic density-93 deficiency protects cultured cortical neurons from N-methyl-D-aspartate receptor-triggered neurotoxicity. Neuroscience 166:1083–1090. https://doi.org/10.1016/j.neuroscience.2010.01.030
Zhang JY, Liu TH, He Y, Pan HQ, Zhang WH, Yin XP et al (2019) Chronic stress remodels synapses in an amygdala circuit-specific manner. Biol Psychiatry 85:189–201. https://doi.org/10.1016/j.biopsych.2018.06.019
Zhao J, Verwer RWH, Gao SF, Qi XR, Lucassen PJ, Kessels HW et al (2018) Prefrontal alterations in GABAergic and glutamatergic gene expression in relation to depression and suicide. J Psychiatr Res 102:261–274. https://doi.org/10.1016/j.jpsychires.2018.04.020
Zhou JJ, Gao Y, Zhang X, Kosten TA, Li DP (2018) Enhanced hypothalamic NMDA receptor activity contributes to hyperactivity of hpa axis in chronic stress in male rats. Endocrinology 159:1537–1546. https://doi.org/10.1210/en.2017-03176
Zhu J, Shang Y, Zhang M (2016) Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat Rev Neurosci 17:209–223. https://doi.org/10.1038/nrn.2016.18
Acknowledgements
This work was supported by National Natural Science Foundation of China (91732304 and 32030046), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB02030001 and XDB32020200). We are grateful to the Netherlands Brain Bank for providing human brain material and clinical details. We thank Dr. Zhi Zhang (USTC) for providing the Cx3cr1-CreERT2 mice.
Author information
Authors and Affiliations
Contributions
J-NZ designed the studies. X-YQ and Q-HS performed most of the experiments and data analysis, and wrote the draft manuscript. HF, YW, and PC conducted some of the molecular experiments and behavioral experiments. Z-QX provided the plasmid. DS provided the human brain tissue and was involved in the revision of the manuscript. X-YQ and J-NZ wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors claim that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 32145 kb)
Rights and permissions
About this article
Cite this article
Qin, XY., Shan, QH., Fang, H. et al. PSD-93 up-regulates the synaptic activity of corticotropin-releasing hormone neurons in the paraventricular nucleus in depression. Acta Neuropathol 142, 1045–1064 (2021). https://doi.org/10.1007/s00401-021-02371-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02371-7