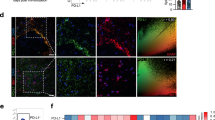
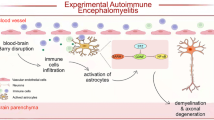
Abstract
Single-nucleotide polymorphisms in the tumor necrosis factor, alpha-induced protein 3 gene, which encodes the ubiquitin-modifying protein A20, are linked to susceptibility to multiple sclerosis (MS), a demyelinating autoimmune disease of the central nervous system (CNS). Since it is unresolved how A20 regulates MS pathogenesis, we examined its function in a murine model of MS, namely experimental autoimmune encephalomyelitis (EAE). Deletion of A20 in neuroectodermal cells (astrocytes, neurons, and oligodendrocytes; Nestin-Cre A20fl/fl mice) or selectively in astrocytes (GFAP-Cre A20fl/fl mice) resulted in more severe EAE as compared to control animals. In Nestin-Cre A20fl/fl and GFAP-Cre A20fl/fl mice demyelination and recruitment of inflammatory leukocytes were increased as compared to A20fl/fl control mice. Importantly, numbers of encephalitogenic CD4+ T cells producing interferon (IFN)-γ, interleukin (IL)-17, and granulocyte–macrophage colony-stimulating factor (GM-CSF), respectively, as well as mRNA production of IFN-γ, IL-17, tumor necrosis factor (TNF), GM-CSF, IL-6, CXCL1, CCL2, and CXCL10 were significantly increased in spinal cords of Nestin-Cre A20fl/fl and GFAP-Cre A20fl/fl mice, respectively. Compared to A20-sufficient astrocytes, A20-deficient astrocytes displayed stronger activation of nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) in response to TNF, IL-17, and GM-CSF, and of signal transducer and activator of transcription 1 (STAT1) upon IFN-γ stimulation. Due to NF-κB and STAT1 hyperactivation, A20-deficient astrocytes produced significantly more chemokines in response to these key encephalitogenic cytokines of autoimmune CD4+ T cells resulting in an amplification of CD4+ T cell recruitment to the CNS. Thus, astrocytic A20 is an important inhibitor of autoimmune-mediated demyelination in the CNS.







Similar content being viewed by others
References
Anderton SM, Liblau RS (2008) Regulatory T cells in the control of inflammatory demyelinating diseases of the central nervous system. Curr Opin Neurol 21(3):248–254
Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH (2002) Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol 22(14):5100–5113
Baxter AG (2007) The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol 7(11):904–912
Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR (2009) Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol 182(5):2628–2640
Brück W, Pförtner R, Pham T, Zhang J, Hayardeny L, Piryatinsky V, Hanisch UK, Regen T, van Rossum D, Brakelmann L, Hagemeier K, Kuhlmann T, Stadelmann C, John GR, Kramann N, Wegner C (2012) Reduced astrocytic NF-kappaB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol 124(3):411–424
Brüstle A, Brenner D, Knobbe CB, Lang PA, Virtanen C, Hershenfield BM, Reardon C, Lacher SM, Ruland J, Ohashi PS, Mak TW (2012) The NF-kappaB regulator MALT1 determines the encephalitogenic potential of Th17 cells. J Clin Invest 122(12):4698–4709
Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, Lauer M, Hascall V, Misra S, Chance MR, Aronica M, Hamilton T, Li X (2011) The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol 12(9):844–852
Chu Y, Vahl JC, Kumar D, Heger K, Bertossi A, Wojtowicz E, Soberon V, Schenten D, Mack B, Reutelshöfer M, Beyaert R, Amann K, van Loo G, Schmidt-Supprian M (2011) B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood 117(7):2227–2236
Codarri L, Gyülveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B (2011) RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12(6):560–567
De Jager PL, Jia X, Wang J, de Bakker PI, Ottoboni L, Aggarwal NT, Piccio L, Raychaudhuri S, Tran D, Aubin C, Briskin R, Romano S, Baranzini SE, McCauley JL, Pericak-Vance MA, Haines JL, Gibson RA, Naeglin Y, Uitdehaag B, Matthews PM, Kappos L, Polman C, McArdle WL, Strachan DP, Evans D, Cross AH, Daly MJ, Compston A, Sawcer SJ, Weiner HL, Hauser SL, Hafler DA, Oksenberg JR (2009) Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet 41(7):776–782
Decker T, Kovarik P (2000) Serine phosphorylation of STATs. Oncogene 19(21):2628–2637
Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G (2010) Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One 5(11):e15531
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36(2):180–190
Engelhardt B, Sorokin L (2009) The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31(4):497–511
Fung EY, Smyth DJ, Howson JM, Cooper JD, Walker NM, Stevens H, Wicker LS, Todd JA (2009) Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun 10(2):188–191
Gilli F, Navone ND, Perga S, Marnetto F, Caldano M, Capobianco M, Pulizzi A, Malucchi S, Bertolotto A (2011) Loss of braking signals during inflammation: a factor affecting the development and disease course of multiple sclerosis. Arch Neurol 68(7):879–888
Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Müller U (2001) Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31(3):367–379
Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA, Reizis B, DeFranco A, Criswell LA, Nakamura MC, Ma A (2011) Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol 12(12):1184–1193
Händel U, Brunn A, Drögemüller K, Müller W, Deckert M, Schlüter D (2012) Neuronal gp130 expression is crucial to prevent neuronal loss, hyperinflammation, and lethal course of murine Toxoplasma encephalitis. Am J Pathol 181(1):163–173
Haroon F, Drögemüller K, Händel U, Brunn A, Reinhold D, Nishanth G, Mueller W, Trautwein C, Ernst M, Deckert M, Schlüter D (2011) Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J Immunol 186(11):6521–6531
Hövelmeyer N, Reissig S, Xuan NT, Adams-Quack P, Lukas D, Nikolaev A, Schlüter D, Waisman A (2011) A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur J Immunol 41(3):595–601
International Multiple Sclerosis Genetics Conssortium (IMSGC) (2010) IL12A, MPHOSPH9/CDK2AP1 and RGS1 are novel multiple sclerosis susceptibility loci. Genes Immun 11(5):397–405
Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, Li X (2010) Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32(3):414–425
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–1175
Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, van Praet J, Branco-Madeira F, Janssens S, Reizis B, Elewaut D, Beyaert R, Hammad H, Lambrecht BN (2011) The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35(1):82–96
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201(2):233–240
Le DG, Kular L, Nicot AB, Calmel C, Melik-Parsadaniantz S, Kitabgi P, Laurent M, Martinerie C (2010) NOV/CCN3 upregulates CCL2 and CXCL1 expression in astrocytes through beta1 and beta5 integrins. Glia 58(12):1510–1521
Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A (2000) Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289(5488):2350–2354
Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH (2008) Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med 205(11):2633–2642
Linden M, Khademi M, Lima Bomfim I, Piehl F, Jagodic M, Kockum I, Olsson T (2013) Multiple sclerosis risk genotypes correlate with an elevated cerebrospinal fluid level of the suggested prognostic marker CXCL13. Mult Scler 19(7):863–870
Liu Y, Teige I, Birnir B, Issazadeh-Navikas S (2006) Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med 12(5):518–525
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408
Ma A, Malynn BA (2012) A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 12(11):774–785
Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Mc Guire C, Vereecke L, Chu Y, Boon L, Staelens S, Matthys P, Lambrecht BN, Schmidt-Supprian M, Pasparakis M, Elewaut D, Beyaert R, van Loo G (2011) A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet 43(9):908–912
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8(9):913–919
McGeachy MJ, Stephens LA, Anderton SM (2005) Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol 175(5):3025–3032
Meads MB, Li ZW, Dalton WS (2010) A novel TNF receptor-associated factor 6 binding domain mediates NF-kappa B signaling by the common cytokine receptor beta subunit. J Immunol 185(3):1606–1615
Miljkovic D, Timotijevic G, Mostarica SM (2011) Astrocytes in the tempest of multiple sclerosis. FEBS Lett 585(23):3781–3788
Musone SL, Taylor KE, Lu TT, Nititham J, Ferreira RC, Ortmann W, Shifrin N, Petri MA, Kamboh MI, Manzi S, Seldin MF, Gregersen PK, Behrens TW, Ma A, Kwok PY, Criswell LA (2008) Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat Genet 40(9):1062–1064
Musone SL, Taylor KE, Nititham J, Chu C, Poon A, Liao W, Lam ET, Ma A, Kwok PY, Criswell LA (2011) Sequencing of TNFAIP3 and association of variants with multiple autoimmune diseases. Genes Immun 12(3):176–182
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65(17):2702–2720
Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR (2009) Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 41(2):199–204
Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H (1994) Patterns of oligodendroglia pathology in multiple sclerosis. Brain 117(Pt 6):1311–1322
Petermann F, Korn T (2011) Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett 585(23):3747–3757
Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F (2009) C–C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10(5):514–523
Shembade N, Ma A, Harhaj EW (2010) Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327(5969):1135–1139
Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ (2011) Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell 44(4):559–571
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM (2008) Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med 14(3):337–342
Tavares RM, Turer EE, Liu CL, Advincula R, Scapini P, Rhee L, Barrera J, Lowell CA, Utz PJ, Malynn BA, Ma A (2010) The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity 33(2):181–191
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23(1):99–103
van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR, Pasparakis M (2006) Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol 7(9):954–961
Varinou L, Ramsauer K, Karaghiosoff M, Kolbe T, Pfeffer K, Müller M, Decker T (2003) Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity 19(6):793–802
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29(37):11511–11522
Vucic D, Dixit VM, Wertz IE (2011) Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol 12(7):439–452
Wang X, Haroon F, Karray S, Martina D, Schlüter D (2013) Astrocytic Fas ligand expression is required to induce T-cell apoptosis and recovery from experimental autoimmune encephalomyelitis. Eur J Immunol 43(1):115–124
Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430(7000):694–699
Yuan C, Qi J, Zhao X, Gao C (2012) Smurf1 protein negatively regulates interferon-gamma signaling through promoting STAT1 protein ubiquitination and degradation. J Biol Chem 287(21):17006–17015
Zepp J, Wu L, Li X (2011) IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol 32(5):232–239
Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF (2001) Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev 15(7):859–876
Acknowledgments
The authors thank Elena Fischer, Nadja Schlüter, and Annette Sohnekind for technical assistance. This work was supported by grants of the Deutsche Forschungsgemeinschaft (GRK 1167 to DS; SFB 854/TP5 to DS and MN).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2013_1183_MOESM1_ESM.tif
Online Resource 1 Upregulation of A20 in the spinal cord during EAE mRNA transcription of A20 in the spinal cord was evaluated by quantitative RT-PCR in untreated and MOG35-55-immunized C57BL/6 mice at day 15 and 22 p.i (n = 3). Data are expressed as the increase of A20 mRNA of immunized over unimmunized mice normalized to HPRT (mean + SEM * p < 0.05) (TIFF 118 kb)
401_2013_1183_MOESM2_ESM.tif
Online Resource 2 EAE was not aggravated in Nestin-Cre A20wt/wt mice Clinical scores of Nestin-Cre A20wt/wt (n = 12) and A20wt/wt (n = 11) mice after active immunization with MOG35-55 peptide. Data show the mean clinical scores + SEM (TIFF 195 kb)
401_2013_1183_MOESM3_ESM.tif
Online Resource 3 EAE was not aggravated in GFAP-Cre A20wt/wt mice and Synapsin-Cre A20fl/fl mice a WB analysis for A20 expression in FACS-sorted microglia and cultured neurons from GFAP-Cre A20fl/fl and A20fl/fl mice. b Clinical scores of EAE in GFAP-Cre A20wt/wt (n = 5) and A20wt/wt (n = 5) mice induced by MOG35-55-immunization. Data show the mean clinical scores + SEM. c WB analysis for A20 expression in cultured neurons from neuron-restricted A20 deficient (Synapsin-Cre A20fl/fl) and control (A20fl/fl) mice. The right panel shows the relative quantification of A20 normalized to GAPDH. Data show the mean + SEM. d Clinical scores of EAE in Synapsin-Cre A20fl/fl (n = 8) and A20fl/fl (n = 6) mice induced by MOG35-55-immunization. Data show the mean clinical scores + SEM (TIFF 1013 kb)
401_2013_1183_MOESM4_ESM.tif
Online Resource 4 Normal apoptosis of A20-deficient and -sufficient astrocytes after TNF stimulation Astrocytes isolated from GFAP-Cre A20fl/fl and A20fl/fl mice were treated with 20 or 100 ng/ml TNF, respectively, for 24 h. Apoptosis was detected by PI and Annexin staining (TIFF 583 kb)
401_2013_1183_MOESM5_ESM.tif
Online Resource 5 STAT1 expression was upregulated in astrocytes after A20 siRNA treatment a Astrocytes derived from C57BL/6 mice were transfected with siRNA targeting A20 or nonsense siRNA. Sixty hours later, WB analysis was performed on total cell lysates for A20, STAT1 and GAPDH. b Relative quantification of A20 normalized to GAPDH. Data show the mean + SEM. c Relative quantification of STAT1 normalized to GAPDH. Data show the mean + SEM (TIFF 246 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Deckert, M., Xuan, N.T. et al. Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-κB- and STAT1-dependent chemokine production in astrocytes. Acta Neuropathol 126, 711–724 (2013). https://doi.org/10.1007/s00401-013-1183-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1183-9