Abstract
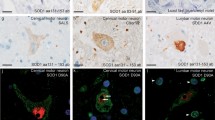
A 46-year-old patient developed amyotrophic lateral sclerosis (ALS) characterized by rapid progression. She needed respiratory assistance after a course of 9 months. She died 4.5 years after onset. Autopsy showed dramatic atrophy of the spinal cord, sparing only the posterior tracts, associated with neuronal loss and astrogliosis in various areas including the anterior horns, motor cortex, striatum, thalamus, and substantia nigra. Ubiquitin immunohistochemistry showed rare skein-like inclusions in the surviving spinal and medullary motor neurons. Eosinophilic inclusions were found in the nuclei of pyramidal neurons in the hippocampus. These inclusions were immunoreactive to antibodies against ubiquitin, promyelocytic leukemia gene product, proteasome, and ataxin-3. They were not immunoreactive to antibodies against tau, cystatin C, neurofilament, alpha-synuclein, SOD-1, and polyglutamine (1C2), and were not stained by ethidium bromide. Similar inclusions were found in the motor cortex. The immunoreactivity of the inclusions was similar to that encountered in diseases associated with CAG repeats, except for the negativity of the immunolabelling with 1C2. At the ultrastructural level, the nuclear inclusions were made of straight filaments (10–12 nm in diameter) arranged at random, reminiscent of the polyglutamine intranuclear hyaline inclusions.


Similar content being viewed by others
References
Bunina T (1962) On intracellular inclusions in familial amyotrophic lateral sclerosis. Korsakov J Neurol Psychiatr 62:1293–1299
Cotter F, Hall P, Young B (1988) Extraction of DNA from small sections of frozen tissue with simultaneous histological examination. J Clin Pathol 41:1125–1126
Daniel M, Koken M, Romagne O, Barbey S, Bazarbachi A, Stadler M (1993) PML protein expression in hematopoietic and acute promyelocytic leukemia cells. Blood 82:1858–1867
Davies S, Beardsall K, Turmaine M, DiFiglia M, Aronin N, Bates G (1998) Are neuronal intranuclear inclusions the common neuropathology of triplet-repeat disorders with polyglutamine-repeat expansions? Lancet 351:131–133
Delatour B, Mercken L, El Hachimi K, Colle M, Pradier L, Duyckaerts C (2001) FE65 in Alzheimer’s disease: neuronal distribution and association with neurofibrillary tangles. Am J Pathol 158:1585–1591
Delisle M, Uro-Coste E, Rascol O, Siani V, Piccardo P, Takao M, Vidal R, Ghetti B (2001) A neurodegenerative disease with intranuclear deposits: clinical and neuropathological studies. J Neuropathol Exp Neurol 60:514
Ferrell K, Wilkinson C, Dubiel W, Gordon C (2000) Regulatory subunit interactions of the 26S proteasome, a complex problem. Trends Biochem Sci 25:83–88
Freemont P (2000) RING for destruction? Curr Biol 10:R84–R87
Fujigasaki H, Uchihara T, Koyano S, Iwabuchi K, Yagishita S, Makifuchi T, Nakamura A, Ishida K, Toru S, Hirai S, Ishikawa K, Tanabe T, Mizusawa H (2000) Ataxin-3 is translocated into the nucleus for the formation of intranuclear inclusions in normal and Machado-Joseph disease brains. Exp Neurol 165:248–256
Fujigasaki H, Uchihara T, Takahashi J, Matsushita H, Nakamura A, Koyano S, Iwabuchi K, Hirai S, Mizusawa H (2001) Preferential recruitment of ataxin-3 independent of expanded polyglutamine: an immunohistochemical study on Marinesco bodies. J Neurol Neurosurg Psychiatr 71:518–520
Funata N, Maeda Y, Koike M, Yano Y, Kaseda M, Muro T, Okeda R, Iwata M, Yokoji M (1990) Neuronal intranuclear hyaline inclusion disease: report of a case and review of the literature. Clin Neuropathol 9:89–96
Greco C, Hagerman R, Tassone F, Chudley A, Del Bigio M, Jacquemont S, Leehey M, Hagerman P (2002) Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125:1760–1771
Gubellini P, Bisso G, Ciofi-Luzzato A, Fortuna S, Lorenzini P, Michalek H, Scarsella G (1997) Ubiquitin-mediated stress response in a rat model of brain transient ischemia/hypoxia. Neurochem Res 22:93–100
Hagerman R, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman P (2001) Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57:127–130
Ince P, Lowe J, Shaw P (1998) Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol Appl Neurobiol 24:104–117
Kakita A, Oyanagi K, Nagai H, Takahashi H (1997) Eosinophilic intranuclear inclusions in the hippocampal pyramidal neurons of a patient with amyotrophic lateral sclerosis. Acta Neuropathol 93:532–536
Leigh P, Whitwell H, Garofalo O, Buller J, Swash M, Martin J, Gallo J, Weller R, Anderton B (1991) Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain 114:775–788
Lieberman A, Robitaille Y, Trojanowski J, Dickson D, Fischbeck K (1998) Polyglutamine-containing aggregates in neuronal intranuclear inclusion disease. Lancet 351:884
Lieberman A, Trojanowski J, Leonard D, Chen K, Barnett J, Leverenz J, Bird T, Robitaille Y, Malandrini A, Fischbeck K (1999) Ataxin 1 and ataxin 3 in neuronal intranuclear inclusion disease. Ann Neurol 46:271–273
Lowe J, Lennox G, Jefferson D, Morrell K, McQuire D, Gray T, Landon M, Doherty F, Mayer R (1988) A filamentous inclusion body within anterior horn neurones in motor neuron disease defined by immunocytochemical localisation of ubiquitin. Neurosci Lett 94:203–210
Mann D, South P, Snowden J, Neary D (1993) Dementia of frontal lobe type. Neuropathology and immunohistochemistry. J Neurol Neurosurg Psychiatr 56:605–614
Matsumoto S, Goto S, Kusaka H, Imai T, Murakami N, Hashizume Y, Okasaki H, Hirano A (1993) Ubiquitin-positive inclusion in anterior horn cells in subgroups of motor neuron disease: a comparative study of adult-onset amyotrophic lateral sclerosis, juvenile amyotrophic lateral sclerosis and Werdnig-Hoffmann disease. J Neurol Sci 115:208–213
Mattsson K, Pokrovskaja K, Kiss C, Klein G, Szekely L (2001) Proteins associated with the promyelocytic leukemia gene product (PML)-containing nuclear body move to the nucleolus upon inhibition of proteasome-dependent protein degradation. Proc Natl Acad Sci USA 98:1012–1017
Okamoto K, Hirai S, Amari M, Iizuka T, Watanabe M, Murakami N, Takatama M (1993) Oculomotor nuclear pathology in amyotrophic lateral sclerosis. Acta Neuropathol 85:458–462
Okamoto K, Hirai S, Amari M, Watanabe M, Sakurai A (1993) Bunina bodies in amyotrophic lateral sclerosis immunostained with rabbit anti-cystatin C serum. Neurosci Lett 162:125–128
Perez M, Paulson H, Pendse S, Saionz S, Bonini N, Pittman R (1998) Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. J Cell Biol 143:1457–1470
Reyes J (2001) PML and COP-1: two proteins with much in common. Trends Biochem Sci 26:18–20
Sasaki S, Tsutsumi Y, Yamane K, Sakuma H, Maruyama S (1992) Sporadic amyotrophic lateral sclerosis with extensive neurological involvement. Acta Neuropathol (Berl) 84:211–215
Schmidt T, Lindenberg K, Krebs A, Schöls L, Laccone F, Herms J, Rechsteiner M, Riess O, Landwehrmeyer G (2002) Protein surveillance machinery in brains with spinocerebellar ataxia type 3: redistribution and differential recruitment of 26S proteasome subunits and chaperones to neuronal intranuclear inclusions. Ann Neurol 51:302–310
Takahashi H, Oyanagi K, Ikuta F, Tanaka M, Yuasa T, Miyatake T (1993) Widespread multiple system degeneration in a patient with familial amyotrophic lateral sclerosis. J Neurol Sci 120:15–21
Takahashi J, Fujigasi H, Zander C, El Hachimi K, Stevanin G, Dürr A, Lebre A, Yvert G, Trottier Y, de Thé H, Hauw J-J, Duyckaerts C, Brice A (2002) Two populations of neuronal intranuclear inclusions in SCA7 differ in size and PML content. Brain 125:1534–1543
Takahashi J, Fukuda T, Tanaka J, Minamitani M, Fujigasaki H, Uchihara T (2000) Neuronal intranuclear hyaline inclusion disease with polyglutamine-immunoreactive inclusions. Acta Neuropathol 99:589–594
Takahashi J, Tanaka J, Arai K, Funata N, Hattori T, Fukuda T, Fujigasaki H, Uchihara T (2001) Recruitment of nonexpanded polyglutamine proteins to intranuclear aggregates in neuronal intranuclear hyaline inclusion disease. J Neuropathol Exp Neurol 60:369–376
Trottier Y, Lutz Y, Stevanin G, Imbert G, Devys D, Cancel G, Saudou F, Weber C, David G, Tora L, Agid Y, Brice A, Mandel J-L (1995) Polyglutamine expansion as a pathological epitope in Huntington’s disease and four dominant cerebellar ataxias. Nature 378:403–406
van Welsem M, Hogenhuis J, Meininger V, Metsaars W, Hauw J-J, Seilhean D (2002) The relationship between Bunina bodies, skein-like inclusions and neuronal loss in amyotrophic lateral sclerosis. Acta Neuropathol 103:583–589
Yamada M, Sato T, Shimohata T, Hayashi S, Igarashi S, Tsuji S, Takahashi H (2001) Interaction between neuronal intranuclear inclusions and promyelocytic leukemia protein nuclear and coiled bodies in CAG repeat diseases. Am J Pathol 159:1785–1795
Zander C, El Hachimi K, Fujigasaki H, Albanese V, Lebre A, Stevanin G, Duyckaerts C, Brice A (2001) Similarities between spinocerebellar ataxia type 7 (SCA7) cell models and human brain: proteins recruited in inclusions and activation of caspase-3. Hum Mol Genet 10:2569–2579
Acknowledgements
J. Takahashi was supported by a fellowship from association Claude Bernard. Special thanks to M. Candau, F. Fierville, O. Freytag, A. Matos, C. N’Ze, C. Py, O. Russaouen, and B. Verneau (Laboratoire de Neuropathologie Raymond Escourolle), and to A. Camuzat (INSERM U289) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seilhean, D., Takahashi, J., El Hachimi, K.H. et al. Amyotrophic lateral sclerosis with neuronal intranuclear protein inclusions. Acta Neuropathol 108, 81–87 (2004). https://doi.org/10.1007/s00401-004-0855-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0855-x