Abstract
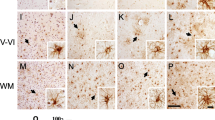
The pathophysiological basis of cognitive dysfunction, including frontotemporal dementia (FTD), in patients with amyotrophic lateral sclerosis (ALS) and ALS with dementia (ALSD) remains unclear. On the other hand, increased expression of cyclooxygenase-2 (COX-2) in the spinal cord is thought to play a pivotal role in motor neuron degeneration in ALS. In this study, to assess the relationship between the neuronal COX-2 expression in the cerebrum, the formation of tau- and α-synuclein-negative but ubiquitin-positive neuronal inclusions (UPIs), and dementia in motor neuron disease (MND), we examined neuronal COX-2 immunoreactivity in the frontal cortex and hippocampus of patients with non-demented ALS without UPIs (n=11), ALSD with UPIs (n=6), and normal controls (n=24) using a quantitative immunohistochemical technique. Neuronal COX-2 expression in all CA1–4 in the hippocampus was significantly up-regulated in the ALSD group, and, to lesser degree but significantly, in the ALS group. Neuronal COX-2 expression in the frontal cortex was also significantly up-regulated in the ALSD group but not in the ALS group. These findings suggest that (1) the frontal cortex and hippocampus of MND are involved in the same pathogenic process associated with COX-2 induction that has been observed in spinal anterior horn cells, (2) COX-2 induction in the cerebrum is a pathogenic process that can occur even in the absence of UPI formation in MND, and (3) COX-2 expression in the cerebrum may be associated with cognitive dysfunction in MND.


Similar content being viewed by others
References
Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, Leigh PN (1996) Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 119:2105–2120
Abrahams S, Goldstein LH, Al-Chalabi A, Pickering A, Morris RG, Passingham RE, Brooks DJ, Leigh PN (1997) Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 62:464–472
Almer G, Guegan C, Teismann P, Naini A, Rosoklija G, Hays AP, Chen C, Przedborski S (2001) Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann Neurol 49:176–185
Arima K, Ueda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, Kawai M (1998) Immunoelectron-microscopic demonstration of NACP/alpha-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson’s disease and in dementia with Lewy bodies. Brain Res 808:93–100
Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Buxbaum JD, Khorkova O, Heroux J, Sahasrabudhe S, Martinez J, Warter JM, Mohr M, Checler F (1997) Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer’s disease and cerebral amyloid angiopathy cases. Mol Med 10:695–707
Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 33:338–357
Drachman DB, Rothstein JD (2000) Inhibition of cyclooxygenase-2 protects motor neurons in an organotypic model of amyotrophic lateral sclerosis. Ann Neurol 48:792–795
Drachman DB, Frank K, Dykes-Hoberg M, Teismann P, Almer G, Przedborski S, Rothstein JD (2002) Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol 52:771–778
Ho L, Purohit D, Haroutunian V, Luterman JD, Willis F, Naslund J, Buxbaum JD, Mohs RC, Aisen PS, Pasinetti GM (2001) Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol 58:487–492
Hoerth-Lomen C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B (2003) Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60:1094–1097
Holton JL, Revesz T, Crooks R, Scaravilli F (2002) Evidence for pathological involvement of the spinal cord in motor neuron disease-inclusion dementia. Acta Neuropathol 103:221–227
Ikeda K, Akiyama H, Arai T, Ueno H, Tsuchiya K, Kosaka K (2002) Morphometrical reappraisal of motor neuron system of Pick’s disease and amyotrophic lateral sclerosis with dementia. Acta Neuropathol 104:21–28
Iwasaki Y, Kinoshita M, Ikeda K, Takamiya K, Shiojima T (1990) Cognitive impairment in amyotrophic lateral sclerosis and its relation to motor disabilities. Acta Neurol Scand 81:141–143
Kawashima T, Doh-ura K, Kikuchi H, Iwaki T (2001) Cognitive dysfunction in patients with amyotrophic lateral sclerosis is associated with spherical or crescent-shaped ubiquitinated intraneuronal inclusions in the parahippocampal gyrus and amygdala, but not in the neostriatum. Acta Neuropathol 102:467–472
Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, Pasinetti GM (1999) Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am J Pathol 155:995–1004
Kew JJ, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, Frackowiak RS, Brooks DJ (1993) The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain 116:1399–1423
Kew JJ, Leigh PN, Playford ED, Passingham RE, Goldstein LH, Frackowiak RS, Brooks DJ (1993) Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain 116:655–680
Mackenzie IRA, Feldman H (2003) The relationship between extramotor ubiquitin-immunoreactive neuronal inclusions and dementia in motor neuron disease. Acta Neuropathol 105:98–102
Mann DM (1998) Dementia of frontal type and dementias with subcortical gliosis. Brain Pathol 8:325–338
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58:1803–1809
Mitsuyama Y, Takamiya S (1979) Presenile dementia with motor neuron disease in Japan. A new entity? Arch Neurol 36:592–593
Nakano I (1993) Temporal lobe lesions in amyotrophic lateral sclerosis with or without dementia—a neuropathological study. Neuropathology 13:215–227
Neary D, Snowden JS, Northen B, Goulding P (1988) Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry 51:353–361
Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N (1990) Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 53:23–32
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Okamoto K, Murakami N, Kusaka H, Yoshida M, Hashizume Y, Nakazato Y, Matsubara E, Hirai S (1992) Ubiquitin-positive intraneuronal inclusions in the extramotor cortices of presenile dementia patients with motor neuron disease. J Neurol 239:426–430
Pasinetti GM, Aisen PS (1998) Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience 87:319–324
Perry TL, Hansen S, Jones K (1987) Brain glutamate deficiency in amyotrophic lateral sclerosis. Neurology 37:1845–1848
Piao YS, Wakabayashi K, Kakita A, Yamada M, Hayashi S, Morita T, Ikuta F, Oyanagi K, Takahashi H (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Plaitakis A, Constantakakis E, Smith J (1988) The neuroexcitotoxic amino acids glutamate and aspartate are altered in the spinal cord and brain in amyotrophic lateral sclerosis. Ann Neurol 24:446–449
Pompl PN, Ho L, Bianchi M, McManus T, Qin W, Pasinetti GM (2003) A therapeutic role for cyclooxygenase-2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. FASEB J 17:725–727
Sairanen T, Ristimaki A, Karjalainen-Lindsberg ML, Paetau A, Kaste M, Lindsberg PJ (1998) Cyclooxygenase-2 is induced globally in infarcted human brain. Ann Neurol 43:738–747
Strauss KI, Marini AM (2002) Cyclooxygenase-2 inhibition protects cultured cerebellar granule neurons from glutamate-mediated cell death. J Neurotrauma 19:627–638
Strong MJ, Grace GM, Orange JB, Leeper HA, Menon RS, Aere C (1999) A prospective study of cognitive impairment in ALS. Neurology 53:1665–1670
Talbot PR (1996) Frontal lobe dementia and motor neuron disease. J Neural Transm Suppl 47:125–132
Talbot PR, Goulding PJ, Lloyd JJ, Snowden JS, Neary D, Testa HJ (1995) Inter-relation between classic motor neuron disease and frontotemporal dementia: neuropsychological and single photon emission computed tomography study. J Neurol Neurosurg Psychiatry 58:541–547
Tan CF, Kakita A, Piao YS, Kikugawa K, Endo K, Tanaka M, Okamoto K, Takahashi H (2003) Primary lateral sclerosis: a rare upper-motor-predominant form of amyotrophic lateral sclerosis often accompanied by frontotemporal lobar degeneration with ubiquitinated neuronal inclusions? Report of an autopsy case and a review of the literature. Acta Neuropathol 105:615–620
The Lund and Manchester Groups (1994) Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 57:416–418
Toyoshima Y, Piao YS, Tan CF, Morita M, Tanaka M, Oyanagi K, Okamoto K, Takahashi H (2003) Pathological involvement of the motor neuron system and hippocampal formation in motor neuron disease inclusion dementia. Acta Neuropathol 106:50–56
Tsuchiya K, Ikeda K, Haga C, Kobayashi T, Morimatsu Y, Nakano I, Matsushita M (2001) Atypical amyotrophic lateral sclerosis with dementia mimicking frontal Pick’s disease: a report of an autopsy case with a clinical course of 15 years. Acta Neuropathol 101:625–630
Tsuchiya K, Ikeda K, Mimura M, Takahashi M, Miyazaki H, Anno M, Shiotsu H, Akabane H, Niizato K, Uchihara T, Tominaga I, Nakano I (2002) Constant involvement of the Betz cells and pyramidal tract in amyotrophic lateral sclerosis with dementia: a clinicopathological study of eight autopsy cases. Acta Neuropathol 104:249–259
Tsuchiya K, Takahashi M, Shiotsu H, Akiyama H, Haga C, Watabiki S, Taki K, Nakano I, Ikeda K (2002) Sporadic amyotrophic lateral sclerosis with circumscribed temporal atrophy: a report of an autopsy case without dementia and with ubiquitinated intraneuronal inclusions. Neuropathology 22:308–316
Wakabayashi K, Piao YS, Hayashi S, Kakita A, Yamada M, Takahashi H (2001) Ubiquitinated neuronal inclusions in the neostriatum in patients with amyotrophic lateral sclerosis with and without dementia—a study of 60 patients 31 to 87 years of age. Clin Neuropathol 20:47–52
Wightman G, Anderson VE, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Wilson CM, Grace GM, Munoz DG, He BP, Strong MJ (2001) Cognitive impairment in sporadic ALS: a pathologic continuum underlying a multisystem disorder. Neurology 57:651–657
Yasojima K, Tourtellotte WW, McGeer EG, McGeer PL (2001) Marked increase in cyclooxygenase-2 in ALS spinal cord: implications for therapy. Neurology 57:952–956
Yokota O, Terada S, Ishizu H, Ishihara T, Ujike H, Nakashima H, Nakashima Y, Kugo A, Checler F, Kuroda S (2003) Cyclooxygenase-2 in the hippocampus is up-regulated in Alzheimer’s disease but not in variant Alzheimer’s disease with cotton wool plaques in humans. Neurosci Lett 343:175–179
Yuasa R (1964) Amyotrophic lateral sclerosis with organic dementia: report of a case. Clin Neurol 4:529–534
Acknowledgements
We would like to thank Mr. M. Kobashi and Ms. M. Onbe for their excellent technical assistance. This work was supported in part by a research grant from the Zikei Institute of Psychiatry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yokota, O., Terada, S., Ishizu, H. et al. Increased expression of neuronal cyclooxygenase-2 in the hippocampus in amyotrophic lateral sclerosis both with and without dementia. Acta Neuropathol 107, 399–405 (2004). https://doi.org/10.1007/s00401-004-0826-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0826-2