Abstract
Dermatomyositis is a rare, type I interferon-driven autoimmune disease, which can affect muscle, skin and internal organs (especially the pulmonary system). In 2021, we have noted an increase in new-onset dermatomyositis compared to the years before the SARS-CoV-2 pandemic in our center. We present four cases of new-onset NXP2 and/or MDA5 positive dermatomyositis shortly after SARS-CoV-2 infection or vaccination. Three cases occurred within days after vaccination with Comirnaty and one case after SARS-CoV-2 infection. All patients required intensive immunosuppressive treatment. MDA5 antibodies could be detected in three patients and NXP2 antibodies were found in two patients (one patient was positive for both antibodies). In this case-based systematic review, we further analyze and discuss the literature on SARS-CoV-2 and associated dermatomyositis. In the literature, sixteen reports (with a total of seventeen patients) of new-onset dermatomyositis in association with a SARS-CoV-2 infection or vaccination were identified. Ten cases occurred after infection and seven after vaccination. All vaccination-associated cases were seen in mRNA vaccines. The reported antibodies included for instance MDA5, NXP2, Mi-2 and TIF1γ. The reviewed literature and our cases suggest that SARS-CoV-2 infection and vaccination may be considered as a potential trigger of interferon-pathway. Consequently, this might serve as a stimulus for the production of dermatomyositis-specific autoantibodies like MDA5 and NXP2 which are closely related to viral defense or viral RNA interaction supporting the concept of infection and vaccination associated dermatomyositis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dermatomyositis is a rare disease with an incidence of 1 to 15 per million [1]. Apart from muscle and skin, the disease can also affect other organs, such as lungs, heart, and blood vessels with varying clinical outcomes, depending on the specific antibody [2]. Although the pathophysiology has not yet been fully elucidated, type I interferon (IFN) is now known to play a key role in the development of the disease. Induction of interferon-stimulated genes can be seen in muscle biopsies of dermatomyositis and type I IFN signature has been reported in peripheral blood samples [3, 4]. Specifically, anti-melanoma differentiation-associated protein 5 (anti-MDA5) antibody-positive dermatomyositis patients showed very high serum type I IFN signature [5].
Interestingly, MDA5 positive dermatomyositis and SARS-CoV-2 infection share clinical and laboratory features, such as inflammatory cytokine profile and interstitial lung involvement [6]. Furthermore, creatine kinase (CK) elevation has been reported in up to 27% of SARS-CoV-2-infected patients [7]. Inflammatory myopathy has been detected in infected patients as well as autoantibody production against nuclear matrix protein-2 (NXP2) and MDA5 without clinical symptoms of dermatomyositis but a correlation of worse pulmonary outcomes [8, 9].
The newly developed messenger ribonucleic acid (mRNA) vaccine is known to induce an IFN signaling, partly also via MDA5 [10]. After SARS-CoV-2 vaccination, elevated IFN levels can be detected in healthy individuals [11]. So far, the development of autoimmune diseases like systemic lupus erythematosus (SLE) [12] and autoimmune myositis [13] after SARS-CoV-2 vaccination have been reported in a few case reports.
Both, SARS-CoV-2 infection and vaccination, may lead to new-onset dermatomyositis via autoimmunity due to interferon signaling, hyperinflammation and autoantibody induction.
Case presentation
We report four cases with the occurrence of MDA5 and/or NXP2 positive dermatomyositis directly linked to SARS-CoV-2 infection or vaccination. Our sample comprises three female and one male patient ranging from 19 to 57 years of age. Three patients experienced the onset of dermatomyositis shortly after SARS-CoV-2 mRNA vaccination with BNT162b2 (Comirnaty) (1–7 days) and one patient 2 weeks after SARS-CoV-2 infection. Intriguingly, patient 1 developed dermatomyositis after his first vaccination, whereas dermatomyositis in patients 3 and 4 evolved after the second vaccination. All patients showed typical skin manifestations and reported proximal myalgia (Fig. 1). Two patients initially presented with arthritis. One patient had severe dyspnea, and another had excessive dysphagia. Only two patients had elevated CK levels. MDA5 antibodies could be detected in three patients and NXP2 antibodies were found in two patients (patient 3 was positive for both antibodies). In three patients, muscle magnetic resonance imaging was performed, showing bilateral proximal myositis. Patient 1, furthermore, developed rapid-progressive interstitial lung disease (RP-ILD). Skin and muscle biopsies showed pathologies consistent with dermatomyositis.
All patients required immunosuppression and were treated with glucocorticoid pulse therapy. Whilst patients 3 and 4 showed mild symptoms that were successfully treated with hydroxychloroquine and azathioprine; patients 1 and 2 had a long hospitalization with multiple intensive care treatments due to life-threatening major organ involvements. Both patients required extensive immunosuppression including ciclosporin A, mycophenolate mofetil and rituximab. Table 1 displays patients’ characteristics and therapeutic concepts.
Moreover, we have noted an increase of dermatomyositis diagnoses in our center since the beginning of the SARS-CoV-2 pandemic with almost a doubling of new-onset dermatomyositis in overall inpatient cases from 0.06 to 0.15% (2017–2020) up to 0.26% in the year 2021 (Table 2).
Methods
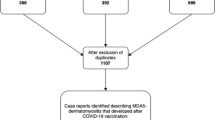
To identify previously reported cases of SARS-CoV-2 associated dermatomyositis, a systematic review of the literature according to PRISMA guidelines was performed. MEDLINE and Embase were systematically searched until the 25th of May 2022. The search strategy included the following terms to identify dermatomyositis cases: ‘myositis’, ‘dermatomyositis’, ‘polymyositis’, ‘rhabdomyolysis’, ‘antisynthetase syndrome’ and ‘inflammatory myopathy’. SARS-CoV-2 association was established with ‘SARS-CoV-2’, ‘COVID-19’ and ‘coronavirus’. All terms were used to search titles and abstracts of publications. The search was conducted as (‘myositis’ OR ‘dermatomyositis’ OR ‘polymyositis’ OR ‘rhabdomyolysis’ OR ‘antisynthetase syndrome’) AND (‘SARS-CoV-2’ OR ‘COVID-19’ OR ‘coronavirus’). The database search in MEDLINE identified 311 publications, the database search in Embase 422, which were independently reviewed by two authors (MTH, NR). A third independent reviewer (MK) decided in case of discrepancy. Based on the EULAR/ACR criteria for (juvenile) dermatomyositis [14], new-onset cases of dermatomyositis with a temporal relation to SARS-CoV-2 infection or vaccination were included in this review. Non-English articles, reviews without description of detailed case information and congress abstracts were excluded. Finally, 16 studies reporting 17 cases were included. The methodology flowchart is shown in Fig. 2.
Results
The clinical, laboratory, radiographic and histopathologic features of SARS-CoV-2 infection-/vaccination-associated dermatomyositis of the identified 17 cases of the systematic review are summarized in Tables 3 and 4 [13, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Interestingly, 70.6% of the patients were female, mean age was 52.4 years. Ten cases occurred after infection and seven after vaccination. All reported vaccinations were mRNA vaccination. Six of these seven cases were after BNT162b2 (Comirnaty) and one after mRNA-1273 (Spikevax) vaccination. All identified cases had pathognomonic skin manifestations. Myocardial involvement was assumed in two cases (one after infection and one after Comirnaty vaccination). Lung involvement was reported in seven patients. Five of these lung involvements were reported after SARS-CoV-2 infection. One patient with MDA5, and two patients with NXP2-antibodies were reported. Furthermore, four Mi-2 positive patients, two RNP/TIF1γ, respectively, and one Jo-1 positive patient were identified. All patients received glucocorticoids and nine patients IVIG. One patient had a lethal disease course.
Discussion
The reported cases vary in autoimmune serology, clinical course, and prognosis. Nevertheless, the common feature was the new-onset dermatomyositis shortly after SARS-CoV-2 infection or vaccination.
Interestingly, lung involvement was the most frequent manifestation (despite skin and muscle). We would like to highlight, that after SARS-CoV-2 infection, radiographically changes of the lung might sometimes be hard to differentiate between infection- or autoimmune-disease related.
In general, viral infections are a well-known trigger of dermatomyositis [30]. Furthermore, seasonal clustering of MDA5-positive dermatomyositis with lower incidence in European summer months is known [31].
In the systematic database search, we identified ten cases of new-onset dermatomyositis after SARS-CoV-2 infection and one patient in our cohort.
In some of these cases apart from classical clinical and laboratory findings of dermatomyositis an IFN signature as well as autoinflammatory clinical aspects have been reported [15, 17, 26].
Consistent with the results of our center, Gokhale et al. also reported an increase of new-onset dermatomyositis in a center in Mumbai with five new cases of dermatomyositis in 6 months from April 2020 (usually one to two new cases per year) [20]. Furthermore, Movahedi et al. described an increase of new-onset juvenile dermatomyositis in Iran. Regularly, two to four new cases were admitted each year from the years 2014 to 2019, whereas from February 2020 to February 2021 eight new-onset juvenile dermatomyositis cases were registered [32].
MDA5- and NXP2-antibodies were reported in each four of the 21 identified cases (16.7%, respectively). Both antibodies are associated with viral interaction in general: MDA5 is an intracellular sensor for viral RNA, triggering proinflammatory immune response especially involving type I IFN [32]. NXP2 shows RNA binding activity and upregulation of its expression has been detected in influenza infection [33]. Furthermore, the two antibodies have been associated with SARS-CoV-2-infections: In a small study of 35 SARS-CoV-2 patients, de Santis et al. reported the occurrence of NXP2 (n = 3) and MDA5 antibodies (n = 1). Both antibodies were associated with a severe disease course [9]. In SARS-CoV-2 infection, MDA5 was shown to guide an innate immune response via IFN signaling [34]. It has been hypothesized, that viral RNA may trigger MDA5 expression and cell damage may lead to MDA5 release followed by autoantibody production [35]. In addition, Wang et al. demonstrated correlative evidence between high titer of anti-MDA5 antibodies and lethal outcome of SARS-CoV-2 infection. Of the 274 patients analyzed, 48.2% were anti-MDA5 positive and high antibody titer (> 10 U/ml) was more frequent in non-survivors [36].
In addition, muscle involvement seems to be an important feature of SARS-CoV-2 infection. Elevated CK was detected in 27% of the SARS-CoV-2 patients [7]. Furthermore, inflammatory myopathy was seen in SARS-CoV-2 patients without significant signs of viral infection of myocytes suggesting autoimmune features [8]. In addition, Manzano et al. discovered the presence of myxovirus resistance protein A (MxA), a type I IFN induced protein, in the muscle biopsy of an SARS-CoV-2 patient with proximal myopathy, suggesting parts of the inflammatory myopathy caused by interferonopathy [38]. Another study also showed immune-mediated and inflammatory myopathy in 16 of 35 autopsies of deceased SARS-CoV-2 patients with high expression of major histocompatibility complex (MHC) I and MxA expression in some cases, which was not seen in controls [39], underlining a possible IFN and cytokine triggered mechanism. These MHC I and IFN patterns found in muscles of SARS-CoV-2 patients closely resemble the pattern found in muscle biopsies in dermatomyositis [2].
Furthermore, the development of autoimmune diseases after vaccination by molecular mimicry and bystander activation in genetically susceptible individuals has frequently been discussed [40, 41]. There have been also a few case reports of vaccinations as a potential trigger of dermatomyositis but no significant association has been established in previous vaccination studies [42].
Rare, but possible side effects after SARS-CoV-2 vaccination, such as the development of autoimmune diseases such as systemic lupus erythematosus (SLE), myocarditis, vasculitis, and thrombotic thrombocytopenia have been reported [12, 43,44,45,46]. In the last few months, since the beginning of the global vaccination campaign, apart from the mentioned autoimmune diseases after vaccination, myositis following SARS-CoV-2 vaccination has been reported. In the reviewed literature and our cohort, we detected ten patients with new-onset dermatomyositis after SARS-CoV-2 vaccination. All patients received a mRNA vaccination. Interestingly, six patients developed the disease after the second vaccination. Whilst mRNA vaccination seems to be more prevalent for dermatomyositis-association, other autoimmune diseases like thrombotic thrombocytopenia or SLE seem more likely to occur after adenovirus vector vaccine like ChAdOx1-S. Autoantibody production and activation is discussed as possible mechanism [12]. Furthermore, there are reports of myositis in temporal association to ChAdOx1-S vaccination [37].
In SARS-CoV-2 mRNA vaccines, the mRNA enters human cells via angiotensin-converting enzyme 2 and induces an immune response to develop spike antibodies against SARS-CoV-2 infection and memory T and B cells [47]. During the development of mRNA vaccine, a strong type I IFN response with MDA5 as one of the possible RNA sensing and IFN inducing mechanisms was seen [10]. Thus, the nowadays used mRNA vaccines are containing nucleoside-modified mRNA, which reduces the IFN pathway activation [10, 48]. Nevertheless, increased type I IFN levels were detected after mRNA vaccination against SARS-CoV-2, but they were comparable to IFN levels after influenza vaccination [11]. As dermatomyositis is known to be an IFN driven disease, there might be a tipping point inducing autoimmunity due to the vaccination response in some patients.
Most recently, Yin et al. were able to prove the importance of the NLRP3 inflammasome in the pathophysiology of dermatomyositis [49]. NLRP3 inflammasome activation has also been detected in myocarditis after mRNA vaccination. It is assumed, that similarly to SARS-CoV-2 infection, spike protein might trigger NLRP3 inflammasome activity, or that the lipid nanoparticles used, might stimulate the NLRP3 inflammasome [50, 51]. This might present another additional pathomechanism in the development of autoimmune diseases like dermatomyositis following mRNA vaccination.
In summary, this case series and the reviewed literature suggest an association between SARS-CoV-2 infection/vaccination and the development of dermatomyositis, since all reported cases occurred within a very short timeframe after vaccination or infection. Possible pathophysiological mechanisms may include type I IFN pathways, the NLRP3 inflammasome and the induction of autoantibody production (especially of those antibodies, which are closely related to viral defense or viral RNA interaction like MDA5 and NXP2).
Due to the limited number of identified cases, we would like to emphasize that the association between SARS-CoV-2 infection/vaccination and the development of dermatomyositis does not necessarily prove causality, and further research is needed.
We would like to underline that the benefit of SARS-CoV-2 vaccinations highly outweighs possible very rare autoimmune phenomena. Nevertheless, rheumatologists should be aware of possible associations between dermatomyositis and SARS-CoV-2 infection/vaccination to maintain optimal medical management.
Availability of data and materials
The data underlying this article cannot be shared publicly due to the anonymization of patients and for the privacy of individuals that participated in this case series. Non-confidential data will be shared on reasonable request to the corresponding author.
References
Kronzer VL, Kimbrough BA, Crowson CS et al (2021) Incidence, prevalence, and mortality of dermatomyositis: a population-based cohort study. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.24786
DeWane ME, Waldman R, Lu J (2020) Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol 82:267–281. https://doi.org/10.1016/j.jaad.2019.06.1309
Greenberg SA, Pinkus JL, Pinkus GS et al (2005) Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol 57:664–678. https://doi.org/10.1002/ana.20464
Baechler EC, Bauer JW, Slattery CA et al (2007) An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 13:59–68. https://doi.org/10.2119/2006-00085.Baechler
Ono N, Kai K, Maruyama A et al (2020) The relationship between type 1 IFN and vasculopathy in anti-MDA5 antibody-positive dermatomyositis patients. Rheumatology (Oxford) 59:918. https://doi.org/10.1093/rheumatology/keaa033
Kondo Y, Kaneko Y, Takei H et al (2021) COVID-19 shares clinical features with anti-melanoma differentiation-associated protein 5 positive dermatomyositis and adult Still’s disease. Clin Exp Rheumatol 39:631–638
Pitscheider L, Karolyi M, Burkert FR et al (2021) Muscle involvement in SARS-CoV-2 infection. Eur J Neurol 28:3411–3417. https://doi.org/10.1111/ene.14564
Aschman T, Schneider J, Greuel S et al (2021) Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol 78:948–960. https://doi.org/10.1001/jamaneurol.2021.2004
de Santis M, Isailovic N, Motta F et al (2021) Environmental triggers for connective tissue disease: the case of COVID-19 associated with dermatomyositis-specific autoantibodies. Curr Opin Rheumatol 33:514–521. https://doi.org/10.1097/BOR.0000000000000844
Pardi N, Hogan MJ, Porter FW et al (2018) mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov 17:261–279. https://doi.org/10.1038/nrd.2017.243
Ntouros PA, Vlachogiannis NI, Pappa M et al (2021) Effective DNA damage response after acute but not chronic immune challenge: SARS-CoV-2 vaccine versus systemic lupus erythematosus. Clin Immunol 229:108765. https://doi.org/10.1016/j.clim.2021.108765
Chen Y, Xu Z, Wang P et al (2021) New-onset autoimmune phenomena post COVID-19 vaccination. Immunology. https://doi.org/10.1111/imm.13443
Vutipongsatorn K, Isaacs A, Farah Z (2022) Inflammatory myopathy occurring shortly after severe acute respiratory syndrome coronavirus 2 vaccination: two case reports. J Med Case Rep 16:57. https://doi.org/10.1186/s13256-022-03266-1
Lundberg IE, Tjärnlund A, Bottai M et al (2017) 2017 European league against rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol 69:2271–2282. https://doi.org/10.1002/art.40320
Keshtkarjahromi M, Chhetri S, Balagani A et al (2021) Macrophage activation syndrome in MDA5 antibody-positive dermatomyositis and COVID-19 infection. BMC Rheumatol 5:59. https://doi.org/10.1186/s41927-021-00225-z
Lee AYS, Lee C, Brown DA et al (2022) Development of anti-NXP2 dermatomyositis following Comirnaty COVID-19 vaccination. Postgrad Med J. https://doi.org/10.1136/postgradmedj-2022-141510
Okada Y, Izumi R, Hosaka T et al (2021) Anti-NXP2 antibody-positive dermatomyositis developed after COVID-19 manifesting as type I interferonopathy. Rheumatology (Oxford). https://doi.org/10.1093/rheumatology/keab872
Ho BVK, Seger EW, Kollmann K et al (2021) Dermatomyositis in a COVID-19 positive patient. JAAD Case Rep 13:97–99. https://doi.org/10.1016/j.jdcr.2021.04.036
Camargo Coronel A, Jiménez Balderas FJ, Quiñones Moya H et al (2022) Dermatomyositis post vaccine against SARS-COV2. BMC Rheumatol 6:20. https://doi.org/10.1186/s41927-022-00250-6
Gokhale Y, Patankar A, Holla U et al (2020) Dermatomyositis during COVID-19 pandemic (a case series): is there a cause effect relationship? J Assoc Physicians India 68:20–24
Gouda W, Albasri A, Alsaqabi F et al (2022) Dermatomyositis following BNT162b2 mRNA COVID-19 vaccination. J Korean Med Sci 37:e32–e32. https://doi.org/10.3346/jkms.2022.37.e32
Wu M, Karim M, Ashinoff R (2022) COVID-19 vaccine associated dermatomyositis. JAAD Case Rep. https://doi.org/10.1016/j.jdcr.2022.02.023
Liquidano-Perez E, García-Romero MT, Yamazaki-Nakashimada M et al (2021) Juvenile dermatomyositis triggered by SARS-CoV-2. Pediatr Neurol 121:26–27. https://doi.org/10.1016/j.pediatrneurol.2021.05.011
Venkateswaran K, Aw DC-W, Huang J et al (2022) Dermatomyositis following COVID-19 vaccination. Dermatol Ther. https://doi.org/10.1111/dth.15479
Shahidi Dadras M, Rakhshan A, Ahmadzadeh A et al (2021) Dermatomyositis-lupus overlap syndrome complicated with cardiomyopathy after SARS-CoV-2 infection: a new potential trigger for musculoskeletal autoimmune disease development. Clin Case Rep 9:e04931–e04931. https://doi.org/10.1002/ccr3.4931
Rodero MP, Pelleau S, Welfringer-Morin A et al (2022) Onset and relapse of juvenile dermatomyositis following asymptomatic SARS-CoV-2 infection. J Clin Immunol 42:25–27. https://doi.org/10.1007/s10875-021-01119-y
Borges NH, Godoy TM, Kahlow BS (2021) Onset of dermatomyositis in close association with COVID-19-a first case reported. Rheumatology (Oxford) 60:SI96. https://doi.org/10.1093/rheumatology/keab290
Derbel A, Guermazi M, El Moctar EM et al (2021) Dermatomyositis following COVID-19 infection. Rev Med Interne 42:A445. https://doi.org/10.1016/j.revmed.2021.10.186
Kreuter A, Lausch S, Burmann S-N et al (2022) Onset of amyopathic dermatomyositis following mRNA-based SARS-CoV-2 vaccination. J Eur Acad Dermatol Venereol n/a. https://doi.org/10.1111/jdv.18211
Bax CE, Maddukuri S, Ravishankar A et al (2021) Environmental triggers of dermatomyositis: a narrative review. Ann Transl Med 9:434. https://doi.org/10.21037/atm-20-3719
Toquet S, Granger B, Uzunhan Y et al (2021) The seasonality of dermatomyositis associated with anti-MDA5 antibody: an argument for a respiratory viral trigger. Autoimmun Rev 20:102788. https://doi.org/10.1016/j.autrev.2021.102788
Movahedi N, Ziaee V (2021) COVID-19 and myositis; true dermatomyositis or prolonged post viral myositis? Pediatr Rheumatol Online J 19:86. https://doi.org/10.1186/s12969-021-00570-w
Ver Lorena S, Marcos-Villar L, Landeras-Bueno S et al (2015) The cellular factor NXP2/MORC3 is a positive regulator of influenza virus multiplication. J Virol 89:10023–10030. https://doi.org/10.1128/JVI.01530-15
Yin X, Riva L, Pu Y et al (2021) MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep 34:108628. https://doi.org/10.1016/j.celrep.2020.108628
Mehta P, Machado PM, Gupta L (2021) Understanding and managing anti-MDA 5 dermatomyositis, including potential COVID-19 mimicry. Rheumatol Int 41:1021–1036. https://doi.org/10.1007/s00296-021-04819-1
Wang G, Wang Q, Wang Y et al (2021) Presence of anti-MDA5 antibody and its value for the clinical assessment in patients with COVID-19: a retrospective cohort study. Front Immunol 12:791348
Gupta K, Sharma GS, Kumar A (2021) COVID-19 vaccination-associated anti-Jo-1 syndrome. Reumatologia 59:420–422. https://doi.org/10.5114/reum.2021.111836
Manzano GS, Woods JK, Amato AA (2020) Covid-19-associated myopathy caused by type I interferonopathy. N Engl J Med 383:2389–2390. https://doi.org/10.1056/NEJMc2031085
Suh J, Mukerji SS, Collens SI et al (2021) Skeletal muscle and peripheral nerve histopathology in COVID-19. Neurology 97:e849. https://doi.org/10.1212/WNL.0000000000012344
Vadalà M, Poddighe D, Laurino C et al (2017) Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J 8:295–311. https://doi.org/10.1007/s13167-017-0101-y
Rodriguez-Pintó I, Shoenfeld Y (2015) Myositis and Vaccines. Wiley Online Books, Hoboken
Orbach H, Tanay A (2009) Vaccines as a trigger for myopathies. Lupus 18:1213–1216. https://doi.org/10.1177/0961203309345734
Abu Mouch S, Roguin A, Hellou E et al (2021) Myocarditis following COVID-19 mRNA vaccination. Vaccine 39:3790–3793. https://doi.org/10.1016/j.vaccine.2021.05.087
Greinacher A, Thiele T, Warkentin TE et al (2021) Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 384:2092–2101. https://doi.org/10.1056/NEJMoa2104840
Garrido I, Lopes S, Simões MS et al (2021) Autoimmune hepatitis after COVID-19 vaccine—more than a coincidence. J Autoimmun 125:102741. https://doi.org/10.1016/j.jaut.2021.102741
Shakoor MT, Birkenbach MP, Lynch M (2021) ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 78:611–613. https://doi.org/10.1053/j.ajkd.2021.06.016
Wack S, Patton T, Ferris LK (2021) COVID-19 vaccine safety and efficacy in patients with immune-mediated inflammatory disease: review of available evidence. J Am Acad Dermatol 85:1274–1284. https://doi.org/10.1016/j.jaad.2021.07.054
Li Y-D, Chi W-Y, Su J-H et al (2020) Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci 27:104. https://doi.org/10.1186/s12929-020-00695-2
Yin X, Han G-C, Jiang X-W et al (2016) Increased expression of the NOD-like receptor family, pyrin domain containing 3 inflammasome in dermatomyositis and polymyositis is a potential contributor to their pathogenesis. Chin Med J (Engl) 129:1047–1052. https://doi.org/10.4103/0366-6999.180528
Theobald SJ, Simonis A, Georgomanolis T et al (2021) Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med 13:e14150. https://doi.org/10.15252/emmm.202114150
Won T, Gilotra NA, Wood MK et al (2022) Increased interleukin 18-dependent immune responses are associated with myopericarditis after COVID-19 mRNA vaccination. Front Immunol 13:851620
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of the manuscript and reviewed and edited the article carefully. The systematic database search was conducted by MTH, MK and NR. The first draft of the manuscript was written by MTH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical Standard
This manuscript does not contain human or animal studies. All patients gave written consent to anonymously publishing their cases, including pictures, in which the patients can’t be identified.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holzer, MT., Krusche, M., Ruffer, N. et al. New-onset dermatomyositis following SARS-CoV-2 infection and vaccination: a case-based review. Rheumatol Int 42, 2267–2276 (2022). https://doi.org/10.1007/s00296-022-05176-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05176-3