Abstract
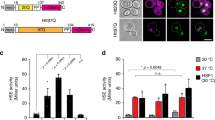
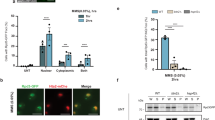
Under thermal stress, different protein quality control (PQC) strategies are activated to maintain an intact proteome, which may vary from one model system to another. Hence thermo-sensitive proteins that lose their active conformation might be refolded with the aid of chaperones or removed by the ubiquitin–proteasome system or the process of autophagy. We have recently developed thermo-sensitive reporters to study PQC in fission yeast and shown the relevance of a third adaptation strategy: the sequestration of misfolded proteins into inclusions which will prevent a rapid degradation and allow the refolding once stress ends. These protein inclusions, protein aggregate centers (PACs), contain a broad spectrum of misfolding/aggregation-prone proteins and chaperones involved in their assembly or dissolution. The chaperone couple Mas5/Ssa2 plays a crucial role in PAC formation, whereas the Hsp104 chaperone promotes their disassembly. The absence of aggregates observed in cells lacking Mas5 could be also explained by the activation of the transcription factor Hsf1 and the induction of chaperone genes, we have excluded this possibility here demonstrating that increased Hsf1 activity and the subsequent overexpression of chaperones do not prevent the assembly of protein aggregates. Protein deposition at certain locations also constitutes a tactic to inactivate proteins temporally. This is the case of Pyp1, the main phosphatase of the stress response kinase Sty1. Upon stress imposition, misfolded Pyp1 is sequestered into cytosolic protein foci while active Sty1 at the nucleus switches on the transcriptional response. In conclusion, we propose that the assembly of aggregation-like foci, PACs in fission yeast, is a crucial PQC strategy during heat stress, and that the Hsp40 chaperone Mas5 is required for PAC assembly and connects physiological and heat-shock triggered PQC.



Similar content being viewed by others
References
Akerfelt M, Morimoto RI, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11:545–555. https://doi.org/10.1038/nrm2938
Boronat S, Marte L, Vega M, García-Santamarina S, Cabrera M, Ayté J, Hidalgo E (2020) The Hsp40 Mas5 connects protein quality control and the general stress reesponse through the thermo-sensitive Pyp1. iScience 23:101725. https://doi.org/10.1016/j.isci.2020.101725
Cabrera M, Boronat S, Marte L, Vega M, Perez P, Ayte J, Hidalgo E (2020) Chaperone-facilitated aggregation of thermo-sensitive proteins shields them from degradation during heat stress. Cell Rep 30(2430–2443):e2434. https://doi.org/10.1016/j.celrep.2020.01.077
Escusa-Toret S, Vonk WIM, Frydman J (2013) Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat Cell Biol 15:1231. https://doi.org/10.1038/ncb2838. https://www.nature.com/articles/ncb2838#supplementary-information
Gaits F, Degols G, Shiozaki K, Russell P (1998) Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast [see comments]. Genes Dev 12:1464–1473
Gonzalez-Medina A, Hidalgo E, Ayte J (2019) Gcn5-mediated acetylation at MBF-regulated promoters induces the G1/S transcriptional wave. Nucleic Acids Res 47:8439–8451. https://doi.org/10.1093/nar/gkz561
Ho CT, Grousl T, Shatz O, Jawed A, Ruger-Herreros C, Semmelink M, Zahn R, Richter K, Bukau B, Mogk A (2019) Cellular sequestrases maintain basal Hsp70 capacity ensuring balanced proteostasis. Nat Commun 10:4851. https://doi.org/10.1038/s41467-019-12868-1
Kaganovich D, Kopito R, Frydman J (2008) Misfolded proteins partition between two distinct quality control compartments. Nature 454:1088–1095. https://doi.org/10.1038/nature07195
Krobitsch S, Lindquist S (2000) Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Natl Acad Sci USA 97:1589–1594
Miller SB, Ho CT, Winkler J, Khokhrina M, Neuner A, Mohamed MY, Guilbride DL, Richter K, Lisby M, Schiebel E, Mogk A, Bukau B (2015) Compartment-specific aggregases direct distinct nuclear and cytoplasmic aggregate deposition. EMBO J 34:778–797. https://doi.org/10.15252/embj.201489524
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796. https://doi.org/10.1101/gad.12.24.3788
Nguyen AN, Shiozaki K (1999) Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev 13:1653–1663
Park SH, Kukushkin Y, Gupta R, Chen T, Konagai A, Hipp MS, Hayer-Hartl M, Hartl FU (2013) PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell 154:134–145. https://doi.org/10.1016/j.cell.2013.06.003
Prouteau M, Loewith R (2018) Regulation of cellular metabolism through phase separation of enzymes. Biomolecules. https://doi.org/10.3390/biom8040160
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266. https://doi.org/10.1016/j.molcel.2010.10.006
Saad S, Cereghetti G, Feng Y, Picotti P, Peter M, Dechant R (2017) Reversible protein aggregation is a protective mechanism to ensure cell cycle restartafter stress. Nat Cell Biol 19:1202–1213. https://doi.org/10.1038/ncb3600
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Shi Y, Mosser DD, Morimoto RI (1998) Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev 12:654–666
Specht S, Miller SB, Mogk A, Bukau B (2011) Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J Cell Biol 195:617–629. https://doi.org/10.1083/jcb.201106037
Spokoini R, Moldavski O, Nahmias Y, England JL, Schuldiner M, Kaganovich D (2012) Confinement to organelle-associated inclusion structures mediates asymmetric inheritance of aggregated protein in budding yeast. Cell Rep 2:738–747. https://doi.org/10.1016/j.celrep.2012.08.024
Tani T, Derby RJ, Hiraoka Y, Spector DL (1996) Nucleolar accumulation of poly (A)+ RNA in heat-shocked yeast cells: implication of nucleolar involvement in mRNA transport. Mol Biol Cell 7:173–192
Vjestica A, Zhang D, Liu J, Oliferenko S (2013) Hsp70-Hsp40 chaperone complex functions in controlling polarized growth by repressing Hsf1-driven heat stress-associated transcription. PLoS Genet 9:e1003886. https://doi.org/10.1371/journal.pgen.1003886
Zhou C, Slaughter BD, Unruh JR, Guo F, Yu Z, Mickey K, Narkar A, Ross RT, McClain M, Li R (2014) Organelle-based aggregation and retention of damaged proteins in asymmetrically dividing cells. Cell 159:530–542. https://doi.org/10.1016/j.cell.2014.09.026
Zuin A, Vivancos AP, Sanso M, Takatsume Y, Ayte J, Inoue Y, Hidalgo E (2005) The glycolytic metabolite methylglyoxal activates Pap1 and Sty1 stress responses in Schizosaccharomyces pombe. J Biol Chem 280:36708–36713
Acknowledgements
This work is supported by the Ministerio de Ciencia, Innovación y Universidades (Spain), PLAN E and FEDER (BFU2016-75116-P to M.C. and PGC2018-093920-B-I00 to E.H.). The Oxidative Stress and Cell Cycle group is also supported by Generalitat de Catalunya (Spain) (2017-SGR-539) and by Unidad de Excelencia María de Maeztu, funded by the AEI (CEX2018-000792-M) (Spain). M.C. is funded by the Ramon y Cajal program (MINECO-RYC2013-12858). E.H. is recipient of an ICREA Academia Award (Generalitat de Catalunya, Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boronat, S., Cabrera, M. & Hidalgo, E. Spatial sequestration of misfolded proteins as an active chaperone-mediated process during heat stress. Curr Genet 67, 237–243 (2021). https://doi.org/10.1007/s00294-020-01135-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-020-01135-2