Abstract
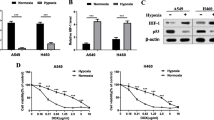
Objectives: Hypoxia is associated with human non-small cell lung cancers (NSCLC), which are highly resistant to chemotherapy. The hypoxia inducible factor (HIF) as a transcription factor in response to hypoxia indicates that it could be a novel, tumor-specific target for anticancer therapy. We hypothesized that disruption of HIF pathway through lentiviral vector-mediated HIF-1α RNA interference (RNAi) could reverse the hypoxia-induced resistance to chemotherapy. Methods: We transfected Human NSCLC cell lines, SPCA1 and A549 with HIF-1α specific RNAi lentiviral vectors as well as controls. HIF-1α silenced cells [SPCA1/HIF-1α(-) and A549/HIF-1α(-)] were screened by blasticidin. They were incubated in 19 or 0.5% O2 for 16 h followed by the assessment of chemosensitivity to cisplatin and doxorubicin with MTT and clonogenic assays. Quantitative RT-PCR and Western blot analysis were used to detect the expressions of HIF-1α mRNA and protein, respectively. Moreover, flow cytometry was used to monitor the expression of P-glycoprotein. Results: Exposure of SPCA1 and A549 cells to 0.5% O2 significantly increased resistance to cisplatin and doxorubicin, in contrast to cells incubated in normoxia. Transduction of SPCA1 with HIF-1α RNAi vector resulted in sequence specific silencing with 87.2 and 84.6% decreases of HIF-1α mRNA transcription and 97.3 and 94.8% of protein expressions in normoxia and hypoxia, respectively. Correspondingly, they are 89.2, 89.9% and 97.2, 88.4% decreases in A549 cells. Hypoxia-induced resistance to cisplatin and doxorubicin were reversed in SPCA1/HIF-1α(-) and A549/HIF-1α(-) cells. There was no significant P-glycoprotein increase induced by hypoxia in NSCLC cells. Conclusions: Our studies demonstrated that hypoxia-induced chemoresistance to cisplatin and doxorubicin in NSCLC cells is through the HIF pathway. MDR1 regulation may not be involved in hypoxia-induced chemoresistance. Combining delivery of HIF-1α RNAi lentiviral vector with cisplatin-related chemotherapy regimens may enable us to develop more effective strategy for NSCLC therapy.




Similar content being viewed by others
References
Belani CP (2002) Chemotherapy regimens in advanced non-small-cell lung cancer: recent randomized trials. Clin Lung Cancer 3(Suppl 1):S5–S9
Arriagada R, Bergman B, Dunant A et al (2004) International adjuvant lung cancer trial collaborative group. N Engl J Med 350:351–360
Kunz M, Ibrahim SM (2003) Molecular responses to hypoxia in tumor cells. Mol Cancer 2(1):23
Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2:38–47
Fedele AO, Whitelaw ML, Peet DJ (2002) Regulation of gene expression by the hypoxia-inducible factors. Mol Interv 2(4):229–243
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. PNAS 92:5510–5514
Manfred Kunz, Saleh M Ibahim (2003) Molecular responses to hypoxia in tumor cells. Mol Cancer 2:23
Claudio J Conti (2002) Vascular endothelial growth factor: regulation I the mouse skin carcinogenesis model and use in antiangiogenesis cancer therapy. Oncologist 7(suppl 3):4–11
Semenza G (2000) HIF-1 and human disease: one highly involved factor. Genes Dev 14(16):1983–1991
Goda N, Ryan HE, Khadivi B et al (2003) Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 23(1):359–369
Postovit LM, Adams MA, Lash GE et al (2002) Oxygen-mediated regulation of tumor cell invasiveness. Involvement of a nitric oxide signaling pathway. J Biol Chem 277:35730
Balaji K, Shannon B-D, Brain K et al (2003) Regulation of colon carcinoma cell invasion by hypoxia-inducible factor. Cancer Res 63:1138–1143
Frederiksen LJ, Siemens DR, Heaton JP et al (2003) Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate. J Urol 170(3):1003–1007
Teicher BA (1994) Hypoxia and drug resistance. Cancer Metastasis Rev 13:139
Wartenberg M, Ling FC, Muschen M et al (2003) Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J 17(3):503–505
Comerford KM, Wallace TJ, Karhausen J et al (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62(12):3387–3394
Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M (2003) Tumor hypoxia: a target for selective cancer therapy. Cancer Sci 94(12):1021–1028
Chen J, Zhao SJ, Nakada K et al (2003) Dominant-negative hypoxia-inducible factor-1α reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am J Pathol 162(4):1283–1291
Kung AL, Wang S, Klco JM et al (2000) Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med 6:1335–1340
Stoeltzing O, McCarty MF, Wey JS et al (2004) Role of hypoxia-inducible factor-1in gastric cancer cell growth, angiogenesis and vessel maturation. JNCI 96:946–956
Unruh A, Ressel A, Mohamed HG et al (2003) The hypoxia-inducible factor-1↑ is a negative factor for tumor therapy. Oncogene 22:3213–3220
Erler JT, Cawthorne CJ, Williams KJ et al (2004) Hypoxia-mediated down-regulation of Bid and Bax in tumours occurs via HIF-1-dependent and-independent mechanisms and contributes to drug resistance. Mol Cell Biol 24:2875–2889
Yeo EJ, Chun YS, Cho YS et al (2003) YC-1 a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst 95:516–525
Michaela S, Michael AM, Matthial E (2003) Gene silencing mediated by small interfering RNAs in mammalian cells. Curr Med Chem 10:245–256
Tuschl T, Borkhardt A (2002) Small interfering RNAs. Mol Interv 2:158–167
Zhang M, Zhang Xin, Bai CX et al (2005) Silencing the epidermal growth factor receptor gene with RNAi may be developed as a potential therapy for non small cell lung cancer. Genet Vaccines Ther 3:5–16
Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW (2004) Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 36(1):1–12
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. PNAS 97(16):9082–9087
Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, Ginouves A, Berra E, Pouyssegur J (2004) HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol 68(6):971–980
Prolonged Hypoxia Differentially Regulates Hypoxia-inducible Factor (HIF)-1_, HIF-2-Expression in Lung Epithelial Cells (2004) J Biol Chem 279(15):14871–14878
Maxwell P, Wiesener M, Chang G-W et al (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275
Kamura T, Sato S, Iwai K et al (2000) Activation of HIF1 alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. PNAS 97(19):10430–10435
Ivan M, Kondo K, Yang H et al (2001) HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292(5516):464–468
Jaideep VT, Gerard P Zambetti, Kazuhiko Arimori, Erin G Schuetz, John D (1997) Schuetz: p53-dependent regulation of MDR1 gene expression causes selective resistance to chemotherapeutic agents. PNAS 94:11037–11042
Acknowledgements
We thank Dr. Fabin Han in Ottawa Health Research Institute, University of Ottawa for revising the manuscript. This work was supported by grants from the National Science Foundation of China (30570547).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, X., Liu, X., Chi, W. et al. Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1α gene. Cancer Chemother Pharmacol 58, 776–784 (2006). https://doi.org/10.1007/s00280-006-0224-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0224-7