Abstract
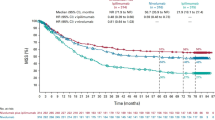
Growing evidence suggests that concurrent loco-regional and systemic treatment modalities may lead to synergistic anti-tumor effects in advanced melanoma. In this retrospective multicenter study, we evaluate the use of electrochemotherapy (ECT) combined with ipilimumab or PD-1 inhibition. We investigated patients with unresectable or metastatic melanoma who received the combination of ECT and immune checkpoint blockade for distant or cutaneous metastases within 4 weeks. Clinical and laboratory data were collected and analyzed with respect to safety and efficacy. A total of 33 patients from 13 centers were identified with a median follow-up time of 9 months. Twenty-eight patients received ipilimumab, while five patients were treated with a PD-1 inhibitor (pembrolizumab n = 3, nivolumab n = 2). The local overall response rate (ORR) was 66.7 %. The systemic ORR was 19.2 and 40.0 % in the ipilimumab and PD-1 cohort, respectively. The median duration of response was not reached in either group. The median time to disease progression was 2.5 months for the entire population with 2 months for ipilimumab and 5 months for PD-1 blockade. The median overall survival was not reached in patients with ipilimumab and 15 months in the PD-1 group. Severe systemic adverse events were detected in 25.0 % in the ipilimumab group. No treatment-related deaths were observed. This is the first reported evaluation of ECT and simultaneous PD-1 inhibition and the largest published dataset on ECT with concurrent ipilimumab. The local response was lower than reported for ECT only. Ipilimumab combined with ECT was feasible, tolerable and showed a high systemic response rate.



Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event(s)
- AJCC:
-
American Joint Committee on Cancer
- CR:
-
Complete response
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- ECT:
-
Electrochemotherapy
- ESOPE:
-
European Standard Operating Procedures for Electrochemotherapy
- LDH:
-
Lactate dehydrogenase
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PD-1:
-
Programmed cell death protein 1
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- SD:
-
Stable disease
- T-VEC:
-
Talimogene laherparepvec
References
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. doi:10.1056/NEJMoa1003466
Robert C, Thomas L, Bondarenko I et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364(26):2517–2526. doi:10.1056/NEJMoa1104621
Schadendorf D, Hodi FS, Robert C et al (2015) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33(17):1889–1894. doi:10.1200/JCO.2014.56.2736
Ribas A, Puzanov I, Dummer R et al (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16(8):908–918. doi:10.1016/S1470-2045(15)00083-2
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372(26):2521–2532. doi:10.1056/NEJMoa1503093
Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, Wolchok JD (2015) Efficacy and safety of nivolumab in patients with BRAF V600 mutant and braf wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol 1(4):433–440. doi:10.1001/jamaoncol.2015.1184
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. doi:10.1056/NEJMoa1412082
Weber JS, D’Angelo SP, Minor D et al (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16(4):375–384. doi:10.1016/S1470-2045(15)70076-8
Thallinger C, Prager G, Ringl H, Zielinski C (2015) Abscopal effect in the treatment of malignant melanoma. Hautarzt 66(7):545–548. doi:10.1007/s00105-014-3567-8
Grimaldi AM, Simeone E, Giannarelli D et al (2014) Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 3:e28780. doi:10.4161/onci.28780 eCollection 2014
Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D (2013) Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol 39(1):4–16. doi:10.1016/j.ejso.2012.08.016
Brizio M, Fava P, Astrua C, Cavaliere G, Savoia P (2015) Complete regression of melanoma skin metastases after electrochemotherapy plus ipilimumab treatment: an unusual clinical presentation. Eur J Dermatol 25(3):271–272. doi:10.1684/ejd.2015.2522
Sersa G, Teissie J, Cemazar M, Signori E, Kamensek U, Marshall G, Miklavcic D (2015) Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol Immunother 64(10):1315–1327. doi:10.1007/s00262-015-1724-2
Queirolo P, Marincola F, Spagnolo F (2014) Electrochemotherapy for the management of melanoma skin metastasis: a review of the literature and possible combinations with immunotherapy. Arch Dermatol Res 306(6):521–526. doi:10.1007/s00403-014-1462-x
Mozzillo N, Simeone E, Benedetto L et al (2015) Assessing a novel immuno-oncology-based combination therapy: ipilimumab plus electrochemotherapy. Oncoimmunology 4(6):e1008842. doi:10.1080/2162402X.2015.1008842
Marty M, Sersa G, Garbay JR et al (2006) Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl 4(11):3–13. doi:10.1016/j.ejcsup.2006.08.002
Campana LG, Clover AJ, Valpione S et al (2016) Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol Oncol 50(1):1–13. doi:10.1515/raon-2016-0006
Balch CM, Gershenwald JE, Soong SJ et al (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27(36):6199–6206. doi:10.1200/JCO.2009.23.4799
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17(4):343–346
Queirolo P, Spagnolo F, Ascierto PA et al (2014) Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 118(1):109–116. doi:10.1007/s11060-014-1400-y
Postow MA, Chesney J, Pavlick AC et al (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372(21):2006–2017. doi:10.1056/NEJMoa1414428
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. doi:10.1056/NEJMoa1504030
Larkin J, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(13):1270–1271. doi:10.1056/NEJMc1509660
Testori A, Rossi CR, Tosti G (2012) Utility of electrochemotherapy in melanoma treatment. Curr Opin Oncol 24(2):155–161. doi:10.1097/CCO.0b013e32834fcaa8
Acknowledgements
We thank Carla Lingner and Diana Lingk for their support with Fig. 1. Bastian Schilling receives research grants from Bristol-Myers Squibb and MSD Sharp and Dohme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Beatrice Schell, Edgar Dippel, Fanny Matheis, Markus V. Heppt, Susanne G. Schäd, and Thilo Gambichler declare no conflict of interest. Bastian Schilling: advisory for Roche, M.S.D. Sharp and Dohme, and Bristol-Myers Squibb, travel support from Roche, Bristol-Myers Squibb and Amgen, honoraria from Roche, M.S.D. Sharp and Dohme and Bristol-Myers Squibb. Carola Berking: advisory for Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche, speaker’s honoraria by Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche. Carmen Loquai: advisory for Roche, Amgen, Novartis, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Ribological, speaker’s honoraria from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, travel reimbursement from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis. Daniela Göppner: advisory for Roche, Amgen, Bristol-Myers Squibb and M.S.D. Sharp and Dohme, speaker’s honoraria from Roche and Bristol-Myers Squibb, travel reimbursement from Roche, Amgen and Novartis. Erwin S. Schultz: advisory for Bristol-Myers Squibb and Novartis, speaker’s honoraria for Novartis. Julia K. Tietze: speaker’s honoraria from Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, Roche. Jens Ulrich: advisory for Roche, speaker’s honoraria from Bristol-Myers Squibb, M.S.D. Sharp and Dohme, GlaxoSmithKline, IGEA, Novartis, Roche, travel reimbursement from Bristol-Myers Squibb, IGEA, Medac, Roche. Katharina C. Kähler: advisory for Roche, Amgen, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, speaker’s honoraria from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, travel reimbursement from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis. Rudolf A. Herbst: advisory for Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Roche, speaker’s honoraria from Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche. Thomas K. Eigentler: advisory for AMGEN, Roche, Bristol-Myers Squibb, travel support from Bristol-Myers Squibb and Novartis, speaker’s honoraria from Roche.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heppt, M.V., Eigentler, T.K., Kähler, K.C. et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother 65, 951–959 (2016). https://doi.org/10.1007/s00262-016-1856-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1856-z