Abstract
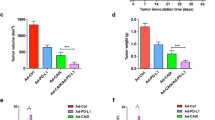
Tumor progression occurs through the modulation of a number of physiological parameters, including the development of immunosuppressive mechanisms to prevent immune detection and response. Among these immune evasion mechanisms, the mobilization of myeloid-derived suppressor cells (MDSC) is a major contributor to the suppression of antitumor T-cell immunity. Patients with renal cell carcinoma (RCC) show increased MDSC, and methods are being explored clinically to reduce the prevalence of MDSC and/or inhibit their function. In the present study, we investigated the relationship between MDSC and the therapeutic potential of a TRAIL-encoding recombinant adenovirus (Ad5-TRAIL) in combination with CpG-containing oligodeoxynucleotides (Ad5-TRAIL/CpG) in an orthotopic mouse model of RCC. This immunotherapy effectively clears renal (Renca) tumors and enhances survival, despite the presence of a high frequency of MDSC in the spleens and primary tumor-bearing kidneys at the time of treatment. Subsequent analyses revealed that the CpG component of the immunotherapy was responsible for decreasing the frequency of MDSC in Renca-bearing mice; further, treatment with CpG modulated the phenotype and function of MDSC that remained after immunotherapy and correlated with an increased T-cell response. Interestingly, the CpG-dependent alterations in MDSC frequency and function did not occur in tumor-bearing mice complicated with diet-induced obesity. Collectively, these data suggest that in addition to its adjuvant properties, CpG also enhances antitumor responses by altering the number and function of MDSC.






Similar content being viewed by others
Abbreviations
- 5-FU:
-
5-Fluorouracil
- Ad5-TRAIL:
-
Recombinant adenovirus encoding TRAIL
- Ag:
-
Antigen
- Batf3:
-
Basic leucine zipper transcription factor, ATF-like 3
- BV650:
-
Brilliant violet 650
- CpG:
-
CpG-containing oligodeoxynucleotide
- DIO:
-
Diet-induced obesity
- HBSS:
-
Hank’s balanced salt solution
- HFF:
-
High-fat feed
- IFN:
-
Interferon
- IL:
-
Interleukin
- i.p.:
-
Intraperitoneal
- IR:
-
Intrarenal
- i.v.:
-
Intravascular
- mAb:
-
Monoclonal antibody
- MACS:
-
Magnet-associated cell sorting
- MDSC:
-
Myeloid-derived suppressor cell
- MHC:
-
Major histocompatibility complex
- PBS:
-
Phosphate-buffered saline
- pDC:
-
Plasmacytoid dendritic cell
- PE:
-
Phycoerytherin
- pfu:
-
Plaque-forming units
- RCC:
-
Renal cell carcinoma
- spDC:
-
Splenic dendritic cells
- TCR:
-
T-cell receptor
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- WT:
-
Wild type
References
Kusmartsev S, Gabrilovich DI (2006) Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother 55:237–245. doi:10.1007/s00262-005-0048-z
Drake CG, Jaffee E, Pardoll DM (2006) Mechanisms of immune evasion by tumors. Adv Immunol 90:51–81. doi:10.1016/S0065-2776(06)90002-9
Mittal D, Gubin MM, Schreiber RD, Smyth MJ (2014) New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 27C:16–25. doi:10.1016/j.coi.2014.01.004
Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59:1593–1600. doi:10.1007/s00262-010-0855-8
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Sem Cancer Biol 22:275–281. doi:10.1016/j.semcancer.2012.01.011
Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA (2010) Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 70:3526–3536. doi:10.1158/0008-5472.CAN-09-3278
Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 69:1553–1560. doi:10.1158/0008-5472.CAN-08-1921
Ochoa AC, Zea AH, Hernandez C, Rodriguez PC (2007) Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 13:721s–726s. doi:10.1158/1078-0432.CCR-06-2197
Fridlender ZG, Sun J, Singhal S, Kapoor V, Cheng G, Suzuki E, Albelda SM (2010) Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther 18:1947–1959. doi:10.1038/mt.2010.159
Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH (2011) Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol 77:12–19. doi:10.1016/j.critrevonc.2010.02.004
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 15:2148–2157. doi:10.1158/1078-0432.CCR-08-1332
Najjar YG, Finke JH (2013) Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol 3:49. doi:10.3389/fonc.2013.00049
Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L (2011) Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem 286:23591–23599. doi:10.1074/jbc.M111.237123
Matsuzaki J, Tsuji T, Chamoto K, Takeshima T, Sendo F, Nishimura T (2003) Successful elimination of memory-type CD8+ T cell subsets by the administration of anti-Gr-1 monoclonal antibody in vivo. Cell Immunol 224:98–105
Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA (2002) Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med 195:517–528
Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F (2010) 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70:3052–3061. doi:10.1158/0008-5472.CAN-09-3690
Mozaffari F, Lindemalm C, Choudhury A, Granstam-Bjorneklett H, Lekander M, Nilsson B, Ojutkangas ML, Osterborg A, Bergkvist L, Mellstedt H (2009) Systemic immune effects of adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide and/or radiotherapy in breast cancer: a longitudinal study. Cancer Immunol Immunother 58:111–120. doi:10.1007/s00262-008-0530-5
Mozaffari F, Lindemalm C, Choudhury A, Granstam-Bjorneklett H, Helander I, Lekander M, Mikaelsson E, Nilsson B, Ojutkangas ML, Osterborg A, Bergkvist L, Mellstedt H (2007) NK-cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radio-therapy. Br J Cancer 97:105–111. doi:10.1038/sj.bjc.6603840
Kemp TJ, Kim J-S, Crist SA, Griffith TS (2003) Induction of necrotic tumor cell death by TRAIL/Apo-2L. Apoptosis 8:587–599
Norian LA, Kresowik TP, Rosevear HM, James BR, Rosean TR, Lightfoot AJ, Kucaba TA, Schwarz C, Weydert CJ, Henry MD, Griffith TS (2012) Eradication of metastatic renal cell carcinoma after adenovirus-encoded TNF-related apoptosis-inducing ligand (TRAIL)/CpG immunotherapy. PLoS One 7:e31085. doi:10.1371/journal.pone.0031085
Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D (2014) Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9:209–222. doi:10.1038/nprot.2014.005
James BR, Tomanek-Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA (2012) Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol 189:1311–1321. doi:10.4049/jimmunol.1100587
Hrushesky WJ, Murphy GP (1973) Investigation of a new renal tumor model. J Surg Res 15:327–336
VanOosten RL, Griffith TS (2007) Activation of tumor-specific CD8+ T Cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res 67:11980–11990
James BR, Brincks EL, Kucaba TA, Boon L, Griffith TS (2014) Effective TRAIL-based immunotherapy requires both plasmacytoid and CD8a DC. Cancer Immunol Immunother 63:685–697
Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM (2000) Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity 13:265–276
Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J (2008) Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 181:346–353
Rocha FG, Chaves KC, Chammas R, Peron JP, Rizzo LV, Schor N, Bellini MH (2010) Endostatin gene therapy enhances the efficacy of IL-2 in suppressing metastatic renal cell carcinoma in mice. Cancer Immunol Immunother 59:1357–1365. doi:10.1007/s00262-010-0865-6
Salup RR, Back TC, Wiltrout RH (1987) Successful treatment of advanced murine renal cell cancer by bicompartmental adoptive chemoimmunotherapy. J Immunol 138:641–647
Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H (2007) The terminology issue for myeloid-derived suppressor cells. Cancer Res 67:425; author reply 426. doi:10.1158/0008-5472.CAN-06-3037
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181:5791–5802
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111:4233–4244. doi:10.1182/blood-2007-07-099226
Kusmartsev S, Gabrilovich DI (2005) STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol 174:4880–4891
Greifenberg V, Ribechini E, Rossner S, Lutz MB (2009) Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol 39:2865–2876. doi:10.1002/eji.200939486
Ueha S, Shand FH, Matsushima K (2011) Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol 11:783–788. doi:10.1016/j.intimp.2011.03.003
Messai Y, Noman MZ, Derouiche A, Kourda N, Akalay I, Hasmim M, Stasik I, Ben Jilani S, Chebil M, Caignard A, Azzarone B, Gati A, Ben Ammar Elgaaied A, Chouaib S (2010) Cytokeratin 18 expression pattern correlates with renal cell carcinoma progression: relationship with Snail. Int J Oncol 36:1145–1154
Shirota Y, Shirota H, Klinman DM (2012) Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol 188:1592–1599. doi:10.4049/jimmunol.1101304
Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S (2007) Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 67:10019–10026. doi:10.1158/0008-5472.CAN-07-2354
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nature Rev Immunol 11:85–97. doi:10.1038/nri2921
Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R (2013) Obesity-driven inflammation and cancer risk: role of myeloid derived suppressor cells and alternately activated macrophages. Am J Cancer Res 3:21–33
Litterman AJ, Zellmer DM, Grinnen KL, Hunt MA, Dudek AZ, Salazar AM, Ohlfest JR (2013) Profound impairment of adaptive immune responses by alkylating chemotherapy. J Immunol 190:6259–6268. doi:10.4049/jimmunol.1203539
Krieg AM (2012) CpG still rocks! Update on an accidental drug. Nucleic Acid Ther 22:77–89. doi:10.1089/nat.2012.0340
Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C (2011) CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res 17:1765–1775. doi:10.1158/1078-0432.CCR-10-2672
Suzuki K, Suda T, Naito T, Ide K, Chida K, Nakamura H (2005) Impaired toll-like receptor 9 expression in alveolar macrophages with no sensitivity to CpG DNA. Am J Respir Crit Care Med 171:707–713. doi:10.1164/rccm.200408-1078OC
Laber DA (2006) Risk factors, classification, and staging of renal cell cancer. Med Oncol 23:443–454. doi:10.1385/MO:23:4:443
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830. doi:10.1172/JCI19451
Lee IS, Shin G, Choue R (2010) Shifts in diet from high fat to high carbohydrate improved levels of adipokines and pro-inflammatory cytokines in mice fed a high-fat diet. Endocrine J 57:39–50
Fenton JI, Nunez NP, Yakar S, Perkins SN, Hord NG, Hursting SD (2009) Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes Obes Metab 11:343–354. doi:10.1111/j.1463-1326.2008.00974.x
Karlsson EA, Sheridan PA, Beck MA (2010) Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr 140:1691–1697. doi:10.3945/jn.110.123653
Karlsson EA, Sheridan PA, Beck MA (2010) Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol 184:3127–3133. doi:10.4049/jimmunol.0903220
Kim CS, Lee SC, Kim YM, Kim BS, Choi HS, Kawada T, Kwon BS, Yu R (2008) Visceral fat accumulation induced by a high-fat diet causes the atrophy of mesenteric lymph nodes in obese mice. Obesity (Silver Spring) 16:1261–1269. doi:10.1038/oby.2008.55
Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57:3239–3246. doi:10.2337/db08-0872
Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA (2009) Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology 126:268–279. doi:10.1111/j.1365-2567.2008.02895.x
Verwaerde C, Delanoye A, Macia L, Tailleux A, Wolowczuk I (2006) Influence of high-fat feeding on both naive and antigen-experienced T-cell immune response in DO10.11 mice. Scand J Immunol 64:457–466. doi:10.1111/j.1365-3083.2006.01791.x
Mito N, Kaburagi T, Yoshino H, Imai A, Sato K (2006) Oral-tolerance induction in diet-induced obese mice. Life Sci 79:1056–1061. doi:10.1016/j.lfs.2006.03.015
Cui J, Xiao Y, Shi YH, Wang B, Le GW (2012) Lipoic acid attenuates high-fat-diet-induced oxidative stress and B-cell-related immune depression. Nutrition 28:275–280. doi:10.1016/j.nut.2011.10.016
Miyazaki Y, Iwabuchi K, Iwata D, Miyazaki A, Kon Y, Niino M, Kikuchi S, Yanagawa Y, Kaer LV, Sasaki H, Onoe K (2008) Effect of high fat diet on NKT cell function and NKT cell-mediated regulation of Th1 responses. Scand J Immunol 67:230–237. doi:10.1111/j.1365-3083.2007.02062.x
Macia L, Delacre M, Abboud G, Ouk TS, Delanoye A, Verwaerde C, Saule P, Wolowczuk I (2006) Impairment of dendritic cell functionality and steady-state number in obese mice. J Immunol 177:5997–6006
Batra A, Okur B, Glauben R, Erben U, Ihbe J, Stroh T, Fedke I, Chang HD, Zeitz M, Siegmund B (2010) Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology 151:56–62. doi:10.1210/en.2009-0565
Tian Z, Sun R, Wei H, Gao B (2002) Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun 298:297–302
Acknowledgments
We thank the University of Iowa Gene Transfer Vector Core for the production of the Ad5-TRAIL vector. This work was supported by a University of Minnesota Doctoral Dissertation Fellowship (BR James), T90DE022732 from the National Institute of Dental & Craniofacial Research (KG Anderson), a Kidney Cancer Association Research Scholarship administered by the American Urological Association (EL Brincks), and the National Institutes of Health Grants AI084913 (D Masopust) and CA109446 (TS Griffith).
Conflict of interest
B. James, K. Anderson, E. Brincks, T. Kucaba, L. Norian, D. Masopust, and T. Griffith declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
James, B.R., Anderson, K.G., Brincks, E.L. et al. CpG-mediated modulation of MDSC contributes to the efficacy of Ad5-TRAIL therapy against renal cell carcinoma. Cancer Immunol Immunother 63, 1213–1227 (2014). https://doi.org/10.1007/s00262-014-1598-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1598-8