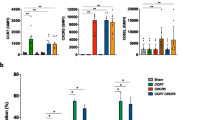
Abstract
Natural killer (NK) cells hold promise for adoptive cancer immunotherapy but are dependent on cytokines such as interleukin (IL)-2 for growth and cytotoxicity. Here, we investigated the consequences of ectopic expression of IL-15 in human NK cells. IL-2 and IL-15 belong to the common γ chain family of cytokines and have overlapping activities. Transduction of clinically applicable NK-92 cells with lentiviral vectors encoding human IL-15 resulted in predominantly intracellular expression of the cytokine, and STAT5 activation, proliferation and cytotoxicity of the producer cells in the absence of IL-2. Growth of non-transduced bystander cells was not supported, allowing rapid enrichment of gene-modified cells solely by IL-2 withdrawal. This was also the case upon transduction of NK-92 and NKL cells with a bicistronic lentiviral vector encoding IL-15 and a chimeric antigen receptor (CAR) targeting the pancarcinoma antigen EpCAM. Effector cells co-expressing CAR and IL-15 continued to proliferate in the absence of exogenous cytokines and displayed high and selective cell-killing activity against EpCAM-expressing breast carcinoma cells that were resistant to the natural cytotoxicity of unmodified NK cells. This strategy facilitates rapid isolation and continuous expansion of retargeted NK cells and may extend their potential clinical utility.






Similar content being viewed by others
References
Gross G, Waks T, Eshhar Z (1989) Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA 86:10024–10028
Uherek C, Groner B, Wels W (2001) Chimeric antigen receptors for the retargeting of cytotoxic effector cells. J Hematother Stem Cell Res 10:523–534. doi:10.1089/15258160152509136
Hombach A, Abken H (2007) Costimulation tunes tumor-specific activation of redirected T cells in adoptive immunotherapy. Cancer Immunol Immunother 56:731–737
Ngo MC, Rooney CM, Howard JM, Heslop HE (2011) Ex vivo gene transfer for improved adoptive immunotherapy of cancer. Hum Mol Genet 20:R93–R99
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725–733. doi:10.1056/NEJMoa1103849
Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, Wels W (2002) Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 100:1265–1273
Wels W, Biburger M, Müller T, Dälken B, Giesübel U, Tonn T, Uherek C (2004) Recombinant immunotoxins and retargeted killer cells: employing engineered antibody fragments for tumor-specific targeting of cytotoxic effectors. Cancer Immunol Immunother 53:217–226. doi:10.1007/s00262-003-0482-8
Imai C, Iwamoto S, Campana D (2005) Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106:376–383
Pegram HJ, Kershaw MH, Darcy PK (2009) Genetic modification of natural killer cells for adoptive cellular immunotherapy. Immunotherapy 1:623–630. doi:10.2217/imt.09.36
Lanier LL (2008) Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 9:495–502. doi:10.1038/ni1581
Klingemann HG (2005) Natural killer cell-based immunotherapeutic strategies. Cytotherapy 7:16–22
Tonn T, Becker S, Esser R, Schwabe D, Seifried E (2001) Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res 10:535–544. doi:10.1089/15258160152509145
Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H (2008) Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 10:625–632. doi:10.1080/14653240802301872
Tavri S, Jha P, Meier R, Henning TD, Müller T, Hostetter D, Knopp C, Johansson M, Reinhart V, Boddington S, Sista A, Wels WS, Daldrup-Link HE (2009) Optical imaging of cellular immunotherapy against prostate cancer. Mol Imaging 8:15–26
Müller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, Tonn T, Wels WS (2008) Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother 57:411–423. doi:10.1007/s00262-007-0383-3
Esser R, Müller T, Stefes D, Kloess S, Seidel D, Gillies SD, Aperlo-Iffland C, Huston JS, Uherek C, Schönfeld K, Tonn T, Huebener N, Lode HN, Koehl U, Wels WS (2011) NK cells engineered to express a GD(2) -specific antigen receptor display built-in ADCC-like activity against tumor cells of neuroectodermal origin. J Cell Mol Med (in press). doi:10.1111/j.1582-4934.2011.01343.x
Maki G, Klingemann HG, Martinson JA, Tam YK (2001) Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res 10:369–383. doi:10.1089/152581601750288975
Overwijk WW, Schluns KS (2009) Functions of gammaC cytokines in immune homeostasis: current and potential clinical applications. Clin Immunol 132:153–165. doi:10.1016/j.clim.2009.03.512
Yamasaki S, Maeda M, Ohshima K, Kikuchi M, Otsuka T, Harada M (2004) Growth and apoptosis of human natural killer cell neoplasms: role of interleukin-2/15 signaling. Leuk Res 28:1023–1031
Zhang J, Sun R, Wei H, Tian Z (2004) Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica 89:338–347
Becknell B, Caligiuri MA (2005) Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 86:209–239. doi:10.1016/S0065-2776(04)86006-1
Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J (1996) Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol 24:406–415
Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B (1988) Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J 7:2089–2095
Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M, Thrasher AJ (2002) High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 13:803–813. doi:10.1089/10430340252898984
Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeister-Wittke U, Plückthun A (1999) High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res 59:5758–5767
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15:871–875. doi:10.1038/nbt0997-871
Zimmermann S, Wels W, Froesch BA, Gerstmayer B, Stahel RA, Zangemeister-Wittke U (1997) A novel immunotoxin recognising the epithelial glycoprotein-2 has potent antitumoural activity on chemotherapy-resistant lung cancer. Cancer Immunol Immunother 44:1–9
Stonier SW, Schluns KS (2010) Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett 127:85–92
Boissel L, Betancur M, Wels WS, Tuncer H, Klingemann H (2009) Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res 33:1255–1259. doi:10.1016/j.leukres.2008.11.024
Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G (2010) Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24:1160–1170. doi:10.1038/leu.2010.75
Chmielewski M, Kopecky C, Hombach AA, Abken H (2011) IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 71:5697–5706. doi:10.1158/0008-5472.CAN-11-0103
Jiang W, Zhang J, Tian Z (2008) Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy 10:265–274. doi:10.1080/14653240801965156
Kurys G, Tagaya Y, Bamford R, Hanover JA, Waldmann TA (2000) The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J Biol Chem 275:30653–30659. doi:10.1074/jbc.M002373200
Bergamaschi C, Jalah R, Kulkarni V, Rosati M, Zhang GM, Alicea C, Zolotukhin AS, Felber BK, Pavlakis GN (2009) Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo. J Immunol 183:3064–3072
Browder TM, Abrams JS, Wong PM, Nienhuis AW (1989) Mechanism of autocrine stimulation in hematopoietic cells producing interleukin-3 after retrovirus-mediated gene transfer. Mol Cell Biol 9:204–213
Konstantinidis KV, Alici E, Aints A, Christensson B, Ljunggren HG, Dilber MS (2005) Targeting IL-2 to the endoplasmic reticulum confines autocrine growth stimulation to NK-92 cells. Exp Hematol 33:159–164. doi:10.1016/j.exphem.2004.11.003
Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, Seliger B, Abken H (2001) Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol 167:6123–6131
Munz M, Baeuerle PA, Gires O (2010) The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res 69:5627–5629. doi:10.1158/0008-5472.CAN-09-0654
van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG (2010) EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis 31:1913–1921. doi:10.1093/carcin/bgq187
Tam YK, Miyagawa B, Ho VC, Klingemann HG (1999) Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J Hematother 8:281–290
Acknowledgments
This work was supported in part by grants from the Bundesministerium für Bildung und Forschung (BMBF) FKZ 01GU0805, the Deutsche Forschungsgemeinschaft (DFG) GRK1172, and the LOEWE Center for Cell and Gene Therapy Frankfurt (CGT). We thank Dr. Uwe Zangemeister-Wittke, University of Bern, for MOC31 antibody, Dr. Manuel Grez, Georg-Speyer-Haus, for helpful discussions and for providing lentiviral vectors and packaging constructs, Dr. Stefan Stein and Mr. Tevik Merovci, Georg-Speyer-Haus for help with flow cytometric cell sorting, and Mrs. Annemarie Schimpf, Georg-Speyer-Haus, for excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sahm, C., Schönfeld, K. & Wels, W.S. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother 61, 1451–1461 (2012). https://doi.org/10.1007/s00262-012-1212-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1212-x