Abstract
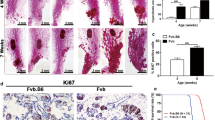
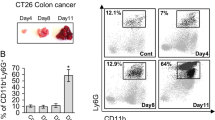
Female mice transgenic for the rat proto-oncogene c-erb-B2, under control of the mouse mammary tumor virus (MMTV) promoter (neuN), spontaneously develop metastatic mammary carcinomas. The development of these mammary tumors is associated with increased number of GR-1+CD11b+ myeloid derived suppressor cells (MDSCs) in the peripheral blood (PB), spleen and tumor. We report a complex relationship between tumor growth, MDSCs and immune regulatory molecules in non-mutated neu transgenic mice on a FVB background (FVB-neuN). The first and second tumors in FVB-neuN mice develop at a median of 265 (147–579) and 329 (161–523) days, respectively, resulting in a median survival time (MST) of 432 (201 to >500) days. During tumor growth, significantly increased number of MDSCs is observed in the PB and spleen, as well as, in infiltrating the mammary tumors. Our results demonstrate a direct correlation between tumor size and the number of MDSCs infiltrating the tumor and an inverse relationship between the frequency of CD4+ T-cells and MDSCs in the spleen. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assessment of enzyme and cytokine transcript levels in the spleen, tumor, tumor-infiltrating non-parenchymal cells (NPCs) and mammary glands revealed a significant increase in transcript levels from grossly normal mammary glands and tumor-infiltrating NPCs during tumor progression. Tumor NPCs, as compared to spleen cells from wild-type (w/t) mice, expressed significantly higher levels of arginase-1 (ARG-1), nitric oxide synthase (NOS-2), vascular endothelial growth factor (VEGF-A) and significantly lower levels of interferon (IFN)-γ, interleukin (IL)-2 and fms-like tyrosine kinase-3 ligand (Flt3L) transcript levels. Transcript levels in the spleens of tumor-bearing (TB) mice also differed from normal mice, although to a lesser extent than transcript levels from tumor-infiltrating NPCs. Furthermore, both spleen cells and NPCs from TB mice, but not control mice, suppressed alloantigen responses by syngeneic control spleen cells. Correlative studies revealed that the number of MDSCs in the spleen was directly associated with granulocyte colony stimulating factor (G-CSF) transcript levels in the spleen; while the number of MDSCs in the tumors was directly correlated with splenic granulocyte macrophage stimulating factor (GM-CSF) transcript levels, tumor volume and tumor cell number. Together our results support a role for MDSCs in tumor initiation and progressive, T-cell depression and loss of function provide evidence which support multiple mechanisms of MDSC expansion in a site-dependent manner.







Similar content being viewed by others
References
Kaplan RN, Psaila B, Lyden D (2006) Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev 25(4):521–529
Ahn GO, Brown JM (2008) Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 13(3):193–205
Seandel M, Butler J, Lyden D, Rafii S (2008) A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization. Cancer Cell 13(3):181–183
Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH et al (2007) Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res 13(16):4840–4848
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W et al (2006) Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 203(12):2691–2702
Serafini P, Mgebroff S, Noonan K, Borrello I (2008) Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68(13):5439–5449
Melani C, Chiodoni C, Forni G, Colombo MP (2003) Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 102(6):2138–2145
Bronte V, Serafini P, Apolloni E, Zanovello P (2001) Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother 24(6):431–446
Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S et al (2007) Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev 128(11–12):672–680
Talmadge JE (2007) Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res 13(18 Pt 1):5243–5248
Gabrilovich D (2004) Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 4(12):941–952
Badger AM, King AG, Talmadge JE, Schwartz DA, Picker DH, Mirabelli CK et al (1990) Induction of non-specific suppressor cells in normal Lewis rats by a novel azaspirane SK&F 105685. J Autoimmun 3(4):485–500
Holda JH, Maier T, Claman HN (1985) Murine graft-versus-host disease across minor barriers: immunosuppressive aspects of natural suppressor cells. Immunol Rev 88:87–105
Strober S (1984) Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol 2:219–237
Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P et al (2000) Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood 96(12):3838–3846
Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA et al (1998) Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol 161(10):5313–5320
Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van EW (1994) Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods 174(1–2):5–19
Gallina G, Dolcetti L, Serafini P, De SC, Marigo I, Colombo MP et al (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116(10):2777–2790
Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J et al (2006) Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66(2):1123–1131
Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N et al (2008) Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 180(12):7898–7906
Rodriguez PC, Ochoa AC (2008) Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev 222:180–191
Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G et al (2004) Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother 53(2):64–72
Donkor M, Lahue E, Hoke T, Shafer L, Coskun U, Solheim JC et al. (2009) Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. [Epub ahead of print]
Talmadge JE, Singh RK, Fidler IJ, Raz A (2007) Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 170(3):793–804
Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B (2007) Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol 7(2):140–151
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Guy CT, Cardiff RD, Muller WJ (1996) Activated neu induces rapid tumor progression. J Biol Chem 271(13):7673–7678
Key ME, Talmadge JE, Fogler WE, Bucana C, Fidler IJ (1982) Isolation of tumoricidal macrophages from lung melanoma metastases of mice treated systemically with liposomes containing a lipophilic derivative of muramyl dipeptide. J Natl Cancer Inst 69(5):1198
Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S et al (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b + Gr1 + myeloid cells. Nat Biotechnol 25(8):911–920
Salomon DS, Brandt R, Ciardiello F, Normanno N (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19(3):183–232
Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P (1989) Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell 57(6):931–936
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ (1992) Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 89(22):10578–10582
Wellings SR, Jensen HM (1973) On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst 50(5):1111–1118
Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P (1988) Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54(1):105–115
Di CE, Diodoro MG, Boggio K, Modesti A, Modesti M, Nanni P et al (1999) Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Lab Invest 79(10):1261–1269
Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C et al (1998) Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med 188(3):589–596
Nanni P, Nicoletti G, De GC, Landuzzi L, Di CE, Iezzi M et al (2003) Prevention of HER-2/neu transgenic mammary carcinoma by tamoxifen plus interleukin 12. Int J Cancer 105(3):384–389
Sas S, Chan T, Sami A, El-Gayed A, Xiang J (2008) Vaccination of fiber-modified adenovirus-transfected dendritic cells to express HER-2/neu stimulates efficient HER-2/neu-specific humoral and CTL responses and reduces breast carcinogenesis in transgenic mice. Cancer Gene Ther 15(10):655–666
Singh R, Paterson Y (2006) Vaccination strategy determines the emergence and dominance of CD8+ T-cell epitopes in a FVB/N rat HER-2/neu mouse model of breast cancer. Cancer Res 66(15):7748–7757
Boggio K, Di CE, Rovero S, Cavallo F, Quaglino E, Lollini PL et al (2000) Ability of systemic interleukin-12 to hamper progressive stages of mammary carcinogenesis in HER2/neu transgenic mice. Cancer Res 60(2):359–364
Cipriani B, Fridman A, Bendtsen C, Dharmapuri S, Mennuni C, Pak I et al (2008) Therapeutic vaccination halts disease progression in BALB-neuT mice: the amplitude of elicited immune response is predictive of vaccine efficacy. Hum Gene Ther 19(7):670–680
Street SE, Zerafa N, Iezzi M, Westwood JA, Stagg J, Musiani P et al (2007) Host perforin reduces tumor number but does not increase survival in oncogene-driven mammary adenocarcinoma. Cancer Res 67(11):5454–5460
Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU (2004) Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene 23(41):6980–6985
Landis MD, Seachrist DD, Abdul-Karim FW, Keri RA (2006) Sustained trophism of the mammary gland is sufficient to accelerate and synchronize development of ErbB2/Neu-induced tumors. Oncogene 25(23):3325–3334
Estrov Z, Talpaz M, Mavligit G, Pazdur R, Harris D, Greenberg SM et al (1995) Elevated plasma thrombopoietic activity in patients with metastatic cancer-related thrombocytosis. Am J Med 98(6):551–558
Kitamura H, Kodama F, Odagiri S, Nagahara N, Inoue T, Kanisawa M (1989) Granulocytosis associated with malignant neoplasms: a clinicopathologic study and demonstration of colony-stimulating activity in tumor extracts. Hum Pathol 20(9):878–885
Ruka W, Rutkowski P, Kaminska J, Rysinska A, Steffen J (2001) Alterations of routine blood tests in adult patients with soft tissue sarcomas: relationships to cytokine serum levels and prognostic significance. Ann Oncol 12(10):1423–1432
Fu YX, Watson G, Jimenez JJ, Wang Y, Lopez DM (1990) Expansion of immunoregulatory macrophages by granulocyte-macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res 50(2):227–234
Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM et al (2008) Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 111(1):219–228
Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P et al (1999) Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 162(10):5728–5737
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S et al (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2(10):1096–1103
Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP et al (1998) Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 160(3):1224–1232
Young MR, Kolesiak K, Wright MA, Gabrilovich DI (1999) Chemoattraction of femoral CD34+ progenitor cells by tumor-derived vascular endothelial cell growth factor. Clin Exp Metastasis 17(10):881–888
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother 58(1):49–59
Ochoa AC, Zea AH, Hernandez C, Rodriguez PC (2007) Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 13(2 Pt 2):721s–726s
Marty M, Pivot X (2008) The potential of anti-vascular endothelial growth factor therapy in metastatic breast cancer: clinical experience with anti-angiogenic agents, focusing on bevacizumab. Eur J Cancer 44(7):912–920
Krishnamurthy S, Sneige N (2002) Molecular and biologic markers of premalignant lesions of human breast. Adv Anat Pathol 9(3):185–197
Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP (2007) Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res 67(23):11438–11446
Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S et al (2007) The terminology issue for myeloid-derived suppressor cells. Cancer Res 67(1):425
Kusmartsev S, Gabrilovich DI (2002) Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother 51(6):293–298
Solheim JC, Reber AJ, Ashour AE, Robinson S, Futakuchi M, Kurz SG et al (2007) Spleen but not tumor infiltration by dendritic and T cells is increased by intravenous adenovirus-Flt3 ligand injection. Cancer Gene Ther 14(4):364–371
Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM (2001) Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol 166(9):5398–5406
Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z et al (2008) Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 181(1):346–353
Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI et al (2000) HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res 60(13):3569–3576
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I (2004) High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 64(17):6337–6343
Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L (2007) Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 18(2):226–232
Reber AJ, Ashour AE, Robinson SN, Talmadge JE, Solheim JC (2004) Flt3 ligand bioactivity and pharmacology in neoplasia. Curr Drug Targets Immune Endocr Metabol Disord 4(2):149–156
Calogero RA, Cordero F, Forni G, Cavallo F (2007) Inflammation and breast cancer. Inflammatory component of mammary carcinogenesis in ErbB2 transgenic mice. Breast Cancer Res 9(4):211
Worschech A, Kmieciak M, Knutson KL, Bear HD, Szalay AA, Wang E et al (2008) Signatures associated with rejection or recurrence in HER-2/neu-positive mammary tumors. Cancer Res 68(7):2436–2446
Astolfi A, Landuzzi L, Nicoletti G, De GC, Croci S, Palladini A et al (2005) Gene expression analysis of immune-mediated arrest of tumorigenesis in a transgenic mouse model of HER-2/neu-positive basal-like mammary carcinoma. Am J Pathol 166(4):1205–1216
Acknowledgments
3419012108027 Avon—Adenovirus p53 Infected DC Vaccine for Breast Cancer. 3132050740—Nebraska Research Initiative—“Translation of Biotechnology into the Clinic”. The authors would like to thank Miss Jill Hallgren for editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abe, F., Dafferner, A.J., Donkor, M. et al. Myeloid-derived suppressor cells in mammary tumor progression in FVB Neu transgenic mice. Cancer Immunol Immunother 59, 47–62 (2010). https://doi.org/10.1007/s00262-009-0719-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0719-2