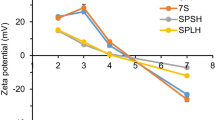
Abstract
Foaming ability of oat protein isolate (OPI) was analysed at pH 4 and 7. Foaming properties were influenced by partial hydrolysis with trypsin (OPT) and alcalase (OPA). The viscoelasticity of the protein film, the interactions between the protein molecules, and the network forming within the protein film were analysed by interfacial rheology. At pH 7, foams made of OPI and OPT were found to be stable with OPI showing the fastest foaming ability. At pH 4, the foaming properties of OPI were found to be poor due to limited solubility. The specific cleavage pattern of trypsin resulted in peptides with improved foaming properties, especially at pH 4, resulting in a homogenous foam structure, a fast foaming ability, and a highly viscoelastic interfacial film. The formation of a thick steric protein layer at pH 7 and the formation of strong hydrophobic interactions at pH 4 were found to be the dominating foam stabilisation mechanisms. In conclusion, oat protein may serve as a food ingredient with targeted functional properties.






Similar content being viewed by others
References
Mohamed A, Biresaw G, Xu J et al (2009) Oats protein isolate: thermal, rheological, surface and functional properties. Food Res Int 42:107–114. https://doi.org/10.1016/j.foodres.2008.10.011
Kaukonen O, Sontag-Strohm T, Salovaara H et al (2011) Foaming of differently processed oats: role of nonpolar lipids and tryptophanin proteins. Cereal Chem 88:239–244. https://doi.org/10.1094/CCHEM-11-10-0154
Konak Üİ, Ercili-Cura D, Sibakov J et al (2014) CO2-defatted oats: solubility, emulsification and foaming properties. J Cereal Sci. https://doi.org/10.1016/j.jcs.2014.01.013
Ma CY, Harwalkar VR (1984) Chemical characterization and functionality assessment of oat protein fractions. J Agric Food Chem 32:144–149. https://doi.org/10.1021/jf00121a035
Guan X, Yao H, Chen Z et al (2007) Some functional properties of oat bran protein concentrate modified by trypsin. Food Chem 101:163–170. https://doi.org/10.1016/j.foodchem.2006.01.011
Cheftel JC, Cuq JL, Lorient D (1992) Chap. 8: Veränderungen von Proteinen. In: Lebensmittelproteine: Biochemie, funktionelle Eigenschaften, Ernährungsphysiologie, chemische Modifizierung. Behr´s Verlag, Hamburg, pp 317–338
Mirmoghtadaie L, Kadivar M, Shahedi M (2009) Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chem 114:127–131. https://doi.org/10.1016/j.foodchem.2008.09.025
Ercili-Cura D, Miyamoto A, Paananen A, Yoshii H (2015) Adsorption of oat proteins to air e water interface in relation to their colloidal state. Food Hydrocoll 44:183–190. https://doi.org/10.1016/j.foodhyd.2014.09.017
Ma CY (1985) Functional properties of oat concentrate treated with linoleate or trypsin. Can Inst Food Sci Technol J 18:79–84. https://doi.org/10.1016/S0315-5463(85)71724-5
Ma CY, Wood DF (1987) Functional properties of oat proteins modified by acylation, trypsin hydrolysis or linoleate treatment. J Am Oil Chem Soc 64:1726–1731. https://doi.org/10.1007/BF02542510
Kilara A, Panyam D (2003) Peptides from milk proteins and their properties. Crit Rev Food Sci Nutr 43:607–633. https://doi.org/10.1080/10408690390251138
Bandyopadhyay K, Misra G, Ghosh S (2008) Preparation and characterisation of protein hydrolysates from indian defatted rice bran meal. J Oleo Sci 57:47–52. https://doi.org/10.5650/jos.57.47
Betancur-Ancona D, Sosa-Espinoza T, Ruiz-Ruiz J et al (2014) Enzymatic hydrolysis of hard-to-cook bean (Phaseolus vulgaris L.) protein concentrates and its effects on biological and functional properties. Int J Food Sci Technol 49:2–8. https://doi.org/10.1111/ijfs.12267
Chabanon G, Chevalot I, Framboisier X et al (2007) Hydrolysis of rapeseed protein isolates: kinetics, characterization and functional properties of hydrolysates. Process Biochem 42:1419–1428. https://doi.org/10.1016/j.procbio.2007.07.009
Condés MC, Scilingo AA, Añón MC (2009) Characterization of amaranth proteins modified by trypsin proteolysis. Structural and functional changes. LWT - Food Sci Technol 42:963–970. https://doi.org/10.1016/j.lwt.2008.12.008
Lqari H, Pedroche J, Girón-Calle J et al (2005) Production of Lupinus angustifolius protein hydrolysates with improved functional properties. Grasas Aceites 56:. https://doi.org/10.3989/gya.2005.v56.i2.121
Molina Ortiz SE, Wagner JR (2002) Hydrolysates of native and modified soy protein isolates: structural characteristics, solubility and foaming properties. Food Res Int 35:511–518. https://doi.org/10.1016/S0963-9969(01)00149-1
Popović L, Peričin D, Vaštag Ž et al (2013) Antioxidative and functional properties of pumpkin oil cake globulin hydrolysates. J Am Oil Chem Soc 90:1157–1165. https://doi.org/10.1007/s11746-013-2257-5
Conde JM, del Mar Yust Escobar M, Pedroche Jiménez JJ et al (2005) Effect of enzymatic treatment of extracted sunflower proteins on solubility, amino acid composition, and surface activity. J Agric Food Chem 53:8038–8045. https://doi.org/10.1021/jf051026i
Wouters AGB, Rombouts I, Legein M et al (2016) Air–water interfacial properties of enzymatic wheat gluten hydrolyzates determine their foaming behavior. Food Hydrocoll 55:155–162. https://doi.org/10.1016/j.foodhyd.2015.11.017
Rodríguez Patino JM, Miñones Conde J, Linares HM et al (2007) Interfacial and foaming properties of enzyme-induced hydrolysis of sunflower protein isolate. Food Hydrocoll 21:782–793. https://doi.org/10.1016/j.foodhyd.2006.09.002
Martínez KD, Carrera Sánchez C, Rodríguez Patino JM, Pilosof AMR (2009) Interfacial and foaming properties of soy protein and their hydrolysates. Food Hydrocoll 23:2149–2157. https://doi.org/10.1016/j.foodhyd.2009.03.015
Conde JM, Rodríguez Patino JM, Trillo JM (2005) Structural characteristics of hydrolysates of proteins from extracted sunflower flour at the air–water interface. Biomacromology 6:3137–3145. https://doi.org/10.1021/bm050469s
Kaukovirta-Norja A, Myllymäki O, Aro H, Hietaniemi V, Pihlava JM, Valtion Teknillinen T (2008) Method for fractionating oat, products thus obtained, and use thereof, WO/2008/096044
Adler-Nissen J (1986) Enzymic hydrolysis of food proteins, 1 st. Elsevier Applied Science Publishers, New York (USA)
Böttcher S, Drusch S (2016) Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophys 11:91–100. https://doi.org/10.1007/s11483-015-9420-5
Benjamins J, Lyklema J, Lucassen-Reynders EH (2006) Compression/expansion rheology of oil/water interfaces with adsorbed proteins. Comparison with the air/water surface. Langmuir 22:6181–6188. https://doi.org/10.1021/la060441h
Ferry JD (1980) Viscoelastic properties of polymers. Wiley, Hoboken
Tschoegl NW (1989) The phenomenological theory of linear viscoelastic behavior. Springer, Berlin
Peterson DM (1978) Subunit structure and composition of oat seed globulin. Plant Physiol 62:506–509. https://doi.org/10.1104/pp.62.4.506
Nivala O, Mäkinen OE, Kruus K et al (2017) Structuring colloidal oat and faba bean protein particles via enzymatic modification. Food Chem 231:87–95. https://doi.org/10.1016/j.foodchem.2017.03.114
Loponen J, Laine P, Sontag-Strohm T, Salovaara H (2007) Behaviour of oat globulins in lactic acid fermentation of oat bran. Eur Food Res Technol 225:105–110. https://doi.org/10.1007/s00217-006-0387-9
Morrissey PA, Mulvihill DM, O’Neill EM (1987) Functional properties of muscle proteins. In: Hudson BJF (ed) Development in food proteins. Elsevier Applied Science, London, pp 195–265
Chen J, Tian J, Zheng F et al (2012) Effects of protein hydrolysis on pasting properties of wheat flour. Starch - Stärke 64:524–530. https://doi.org/10.1002/star.201100180
Plietz P, Zirwer D, Schlesier B et al (1984) Shape, symmetry, hydration and secondary structure of the legumin from Vicia faba in solution. Biochim Biophys Acta Protein Struct Mol Enzymol 784:140–146. https://doi.org/10.1016/0167-4838(84)90120-1
Nieto-Nieto TV, Wang YX, Ozimek L, Chen L (2014) Effects of partial hydrolysis on structure and gelling properties of oat globular proteins. Food Res Int 55:418–425. https://doi.org/10.1016/j.foodres.2013.11.038
Osman A, El-Araby G, Taha H (2016) Potential use as a bio-preservative from lupin protein hydrolysate generated by alcalase in food system. J Appl Biol Biotechnol. https://doi.org/10.7324/JABB.2016.40212
Svendsen I, Breddam K (1992) Isolation and amino acid sequence of a glutamic acid specific endopeptidase from Bacillus licheniformis. Eur J Biochem 204:165–171. https://doi.org/10.1111/j.1432-1033.1992.tb16619.x
Brinegar AC, Peterson DM (1982) Separation and characterization of oat globulin polypeptides. Arch Biochem Biophys 219:71–79. https://doi.org/10.1016/0003-9861(82)90135-7
Burgess SR, Shewry PR, Matlashewski GJ et al (1983) Characteristics of oat (Avena sativa L.) seed globulins. J Exp Bot 34:1320–1332. https://doi.org/10.1093/jxb/34.10.1320
Liu G, Li J, Shi K et al (2009) Composition, secondary structure, and self-assembly of oat protein isolate. J Agric Food Chem 57:4552–4558. https://doi.org/10.1021/jf900135e
Drago SR, González RJ (2000) Foaming properties of enzymatically hydrolysed wheat gluten. Innov Food Sci Emerg Technol 1:269–273. https://doi.org/10.1016/S1466-8564(00)00034-5
Kong X, Zhou H, Qian H (2007) Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chem 102:759–763. https://doi.org/10.1016/j.foodchem.2006.06.062
Lqari H, Pedroche J, Girón-Calle J et al (2005) Production of lupinus angustifolius protein hydrolysates with improved functional properties. Grasas Aceites 56:135–140
Pedroche J, Yust M, Lqari H et al (2004) Brassica carinata protein isolates: chemical composition, protein characterization and improvement of functional properties by protein hydrolysis. Food Chem 88:337–346. https://doi.org/10.1016/j.foodchem.2004.01.045
Yust M, Pedroche M, J, Millán-Linares M del C, et al (2010) Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chem 122:1212–1217. https://doi.org/10.1016/j.foodchem.2010.03.121
Panyam D, Kilara A (1996) Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci Technol 7:120–125. https://doi.org/10.1016/0924-2244(96)10012-1
Zhang Z, Li G, Shi B (2006) Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J Soc Leather Technol Chem 90:23–28
Jung S, Murphy PA, Johnson LA (2005) Physicochemical and functional properties of soy protein substrates modified by low levels of protease hydrolysis. J Food Sci 70:C180–C187. https://doi.org/10.1111/j.1365-2621.2005.tb07080.x
Tsumura K, Saito T, Tsuge K et al (2005) Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT Food Sci Technol 38:255–261. https://doi.org/10.1016/j.lwt.2004.06.007
De Jongh HHJ, Kosters HA, Kudryashova E et al (2004) Protein adsorption at air–water interfaces: a combination of details. Biopolymers 74:131–135. https://doi.org/10.1002/bip.20036
Kinsella J (1981) Functional properties of proteins: possible relationships between structure and function in foams. Food Chem 7:273–288. https://doi.org/10.1016/0308-8146(81)90033-9
Wilde P (2000) Interfaces: their role in foam and emulsion behaviour. Curr Opin Colloid Interface Sci 5:176–181. https://doi.org/10.1016/S1359-0294(00)00056-X
Damodaran S (2005) R: concise reviews/hypotheses in food science protein stabilization of emulsions and foams. Food Sci 70:54–66
Murray BS (2007) Stabilization of bubbles and foams. Curr Opin Colloid Interface Sci 12:232–241. https://doi.org/10.1016/j.cocis.2007.07.009
Hunter TN, Pugh RJ, Franks GV, Jameson GJ (2008) The role of particles in stabilising foams and emulsions. Adv Colloid Interface Sci 137:57–81. https://doi.org/10.1016/j.cis.2007.07.007
Bos AM, van Vliet T (2001) Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Colloid Interface Sci 91:437–471. https://doi.org/10.1016/S0001-8686(00)00077-4
Mitropoulos V, Mütze A, Fischer P (2014) Mechanical properties of protein adsorption layers at the air/water and oil/water interface: a comparison in light of the thermodynamical stability of proteins. Adv Colloid Interface Sci 206:195–206. https://doi.org/10.1016/j.cis.2013.11.004
Dickinson E (2003) Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll 17:25–39. https://doi.org/10.1016/S0268-005X(01)00120-5
Zhao Y, Mine Y, Ma CY (2004) Study of thermal aggregation of oat globulin by laser light scattering. J Agric Food Chem 52:3089–3096. https://doi.org/10.1021/jf030735y
Turgeon SL, Gauthier SF, Molle D, Leonil J (1992) Interfacial properties of tryptic peptides of beta-lactoglobulin. J Agric Food Chem 40:669–675. https://doi.org/10.1021/jf00016a030
Singh AM, Dalgleish DG (1998) The emulsifying properties of hydrolyzates of whey proteins. J Dairy Sci 81:918–924. https://doi.org/10.3168/jds.S0022-0302(98)75651-6
Freer EM, Yim KS, Fuller GG, Radke CJ (2004) Interfacial rheology of globular and flexible proteins at the hexadecane/water interface: comparison of shear and dilatation deformation. J Phys Chem B 108:3835–3844. https://doi.org/10.1021/jp037236k
Gochev G, Retzlaff I, Exerowa D, Miller R (2014) Electrostatic stabilization of foam films from β-lactoglobulin solutions. Colloids Surf A Physicochem Eng Asp 460:272–279. https://doi.org/10.1016/j.colsurfa.2013.12.037
Damodaran S, Paraf A (1997) Food proteins and their applications. Marcel Dekker Inc., New York
Perez AA, Sánchez CC, Rodríguez Patino JM et al (2012) Effect of enzymatic hydrolysis and polysaccharide addition on the β-lactoglobulin adsorption at the air–water interface. J Food Eng 109:712–720. https://doi.org/10.1016/j.jfoodeng.2011.11.017
Davis JP, Doucet D, Foegeding EA (2005) Foaming and interfacial properties of hydrolyzed β-lactoglobulin. J Colloid Interface Sci 288:412–422. https://doi.org/10.1016/j.jcis.2005.03.002
Tamm F, Sauer G, Scampicchio M, Drusch S (2012) Pendant drop tensiometry for the evaluation of the foaming properties of milk-derived proteins. Food Hydrocoll 27:371–377. https://doi.org/10.1016/j.foodhyd.2011.10.013
Roth S, Murray BS, Dickinson E (2000) Interfacial shear rheology of aged and heat-treated β-lactoglobulin films: displacement by nonionic surfactant. J Agric Food Chem 48:1491–1497. https://doi.org/10.1021/jf990976z
Izmailova VN (1979) Structure formation and rheological properties of proteins and surface polymers of interfacial adsorption layers. Progr Surf Membr Sci 13:141–209
Acknowledgements
The project is part of the ERA-NET SUSFOOD “OATPRO, Engineering of oat proteins: Consumer driven sustainable food development process”. The authors thank the Federal Ministry of Education and Research (BMBF), Germany Projektträger Jülich for the financial support (project no. 031A661). The authors acknowledge Cornelia Rauh and Daniel Baier for assistance in foaming experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals.
Rights and permissions
About this article
Cite this article
Brückner-Gühmann, M., Heiden-Hecht, T., Sözer, N. et al. Foaming characteristics of oat protein and modification by partial hydrolysis. Eur Food Res Technol 244, 2095–2106 (2018). https://doi.org/10.1007/s00217-018-3118-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3118-0