Abstract
Rationale
Once dependent on alcohol or opioids, negative affect may accompany withdrawal. Dependent individuals are hypothesized to “self-medicate” in order to cope with withdrawal, which promotes escalated alcohol and drug use.
Objectives
The current study aimed to develop a reliable animal model to assess symptoms that occur during spontaneous alcohol and opioid withdrawal.
Methods
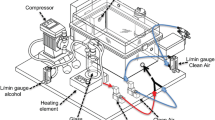
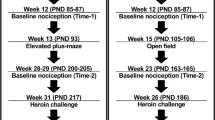
Dependence was induced using intermittent alcohol exposure or pulsatile heroin delivery and assessed for the presence of withdrawal symptoms during acute withdrawal by measuring somatic signs, behavior in the forced swim test (FST), and air-puff-induced 22-kHz ultrasonic vocalizations (USVs). Additional animals subjected to 8 weeks of alcohol vapor exposure were evaluated for altered somatic signs, operant alcohol self-administration, and 22-kHz USV production, as well as performance in the elevated plus maze (EPM).
Results
During spontaneous withdrawal from pulsatile heroin or intermittent alcohol vapor, animals displayed increased somatic withdrawal signs, FST immobility, and 22-kHz USV production but did not show any behavioral change in the EPM unless the duration of alcohol exposure was extended to 4 weeks. Following 8 weeks of alcohol vapor exposure, animals displayed somatic withdrawal signs, escalated alcohol self-administration, and increased 22-kHz USVs.
Conclusions
These paradigms provide consistent methods to evaluate the behavioral ramifications, and neurobiological substrates, of alcohol and opioid dependence during spontaneous withdrawal. As immobility in the FST and percent open-arm time in the EPM were dissociable, with 22-kHz USVs paralleling immobility in the FST, assessment of air-puff-induced 22-kHz USVs could provide an ethologically valid alternative to the FST.






Similar content being viewed by others
References
Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421
Azar MR, Ahmed SH, Lintz R, Gutierrez T, Stinus L, Koob GF (2004) A non-invasive gating device for continuous drug delivery that allows control over the timing and duration of spontaneous opiate withdrawal. J Neurosci Methods 135:129–135
Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004) Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 111:33–51
Barr GA, Zmitrovich A, Hamowy AS, Liu PY, Wang S, Hutchings DE (1998) Neonatal withdrawal following pre- and postnatal exposure to methadone in the rat. Pharmacol Biochem Behav 60:97–104
Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ (2006) The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol 11:270–288
Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL (2011) Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med 12:657–667
Blasig J, Herz A, Reinhold K, Zieglgansberger S (1973) Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia 33:19–38
Breese GR, Overstreet DH, Knapp DJ, Navarro M (2005) Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology 30:1662–1669
Brown SA, Schuckit MA (1988) Changes in depression among abstinent alcoholics. J Stud Alcohol 49:412–417
Burgdorf J, Knutson B, Panksepp J, Shippenberg TS (2001) Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology (Berl) 155:35–42
Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA (2012) Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology 62:167–176
Chen SA, O'Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF (2006) Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31:2692–2707
Cochin J, Miller JM, Rosow CE, Grell R, Poulsen JL (1979) The influence of the mode of morphine administration on tolerance and dependence. NIDA Res Monogr 27:36–47
Contarino A, Papaleo F (2005) The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci USA 102:18649–18654
Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245
Cryan JF, Page ME, Lucki I (2005a) Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 182:335–344
Cryan JF, Valentino RJ, Lucki I (2005b) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569
Devaud LL, Matthews DB, Morrow AL (1999) Gender impacts behavioral and neurochemical adaptations in ethanol-dependent rats. Pharmacol Biochem Behav 64:841–849
Duman CH (2010) Models of depression. Vitam Horm 82:1–21
Fernandes M, Kluwe S, Coper H (1977) Quantitative assessment of tolerance to and dependence on morphine in mice. Naunyn Schmiedebergs Arch Pharmacol 297:53–60
Gellert VF, Holtzman SG (1978) Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther 205:536–546
Getachew B, Hauser SR, Taylor RE, Tizabi Y (2008) Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav 91:97–103
Getachew B, Hauser SR, Taylor RE, Tizabi Y (2010) Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol Biochem Behav 96:395–401
Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL (2011) Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry 69:236–244
Grant BF, Harford TC (1995) Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend 39:197–206
Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF (2009) Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol 14:130–143
Harris GC, Aston-Jones G (2003) Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology 28:292–299
Harwood HJ, Fountain D, Livermore G (1998) Economic costs of alcohol abuse and alcoholism. Recent Dev Alcohol 14:307–330
Hasin DS, Grant BF (2002) Major depression in 6050 former drinkers: association with past alcohol dependence. Arch Gen Psychiatry 59:794–800
Hay JL, Kaboutari J, White JM, Salem A, Irvine R (2010) Model of methadone-induced hyperalgesia in rats and effect of memantine. Eur J Pharmacol 626:229–233
Kalinichev M, Holtzman SG (2003) Changes in urination/defecation, auditory startle response, and startle-induced ultrasonic vocalizations in rats undergoing morphine withdrawal: similarities and differences between acute and chronic dependence. J Pharmacol Exp Ther 304:603–609
Kaplan CD (1992) In: de Vries MW (ed) Drug craving and drug use in the daily life of heroin addicts. Cambridge University Press, Cambridge, pp 193–218
Kliethermes CL (2005) Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev 28:837–850
Knapp DJ, Pohorecky LA (1995) An air-puff stimulus method for elicitation of ultrasonic vocalizations in rats. J Neurosci Methods 62:1–5
Knapp DJ, Kampov-Polevoy AB, Overstreet DH, Breese GR, Rezvani AH (1997) Ultrasonic vocalization behavior differs between lines of ethanol-preferring and nonpreferring rats. Alcohol Clin Exp Res 21:1232–1240
Knapp DJ, Duncan GE, Crews FT, Breese GR (1998) Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res 22:481–493
Knutson B, Burgdorf J, Panksepp J (2002) Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull 128:961–977
Koob GF (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–243
Koob GF (2009) Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42(Suppl 1):S32–S41
Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58
Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Lopez MF, Becker HC (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181:688–696
Lynch HJ, Rivest RW, Wurtman RJ (1980) Artificial induction of melatonin rhythms by programmed microinfusion. Neuroendocrinology 31:106–111
Markou A, Kosten TR, Koob GF (1998) Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18:135–174
Marshall I, Weinstock M (1971) Quantitative method for assessing one symptom of the withdrawal syndrome in mice after chronic morphine administration. Nature 234:223–224
Matsumoto I, Burke L, Inoue Y, Wilce PA (2001) Two models of ethanol withdrawal kindling. Nihon Arukoru Yakubutsu Igakkai Zasshi 36:53–64
Substance Abuse and Mental Health Services Administration (2005) 2005 State estimates of substance use & mental health: substance dependence, abuse, and treatment need. SAMHSA, Rockville, MD
Substance Abuse and Mental Health Services Administration (2010) Results from the 2009 national survey on drug use and health. In: Office of Applied Studies (ed) DHHS, Rockville, MD
Molina VA, Heyser CJ, Spear LP (1994) Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology (Berl) 114:433–440
Moy SS, Knapp DJ, Criswell HE, Breese GR (1997) Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology (Berl) 131:354–360
Moy SS, Knapp DJ, Duncan GE, Breese GR (2000) Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology (Berl) 152:208–215
Mutschler NH, Miczek KA (1998) Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 136:402–408
National Research Council (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington, DC
National Research Council (US) Committee on Guidelines (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academy Press, Washington, DC
Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM (2011) kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61:35–42
Nunes E, Quitkin F, Brady R, Post-Koenig T (1994) Antidepressant treatment in methadone maintenance patients. J Addict Dis 13:13–24
Nutt DJ, Stein DJ (2006) Understanding the neurobiology of comorbidity in anxiety disorders. CNS Spectr 11:13–20
O'Dell LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28:1676–1682
Overstreet DH, Knapp DJ, Breese GR (2004) Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav 78:459–464
Pandey SC, Zhang D, Mittal N, Nayyar D (1999) Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther 288:866–878
Paterson NE, Myers C, Markou A (2000) Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 152:440–446
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Portfors CV (2007) Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci 46:28–34
Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373:2223–2233
Rogers J, Wiener SG, Bloom FE (1979) Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol 27:466–486
Samson HH (1986) Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10:436–442
Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL (1997) Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry 154:948–957
Schulteis G, Markou A, Cole M, Koob GF (1995) Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA 92:5880–5884
Schulteis G, Yackey M, Risbrough V, Koob GF (1998) Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol Biochem Behav 60:727–731
Smith AW, Nealey KA, Wright JW, Walker BM (2011) Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem 96:199–206
Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ (2006) Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp Neurol 202:139–151
Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF (2002) Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res 26:1494–1501
Valenstein ES, Cox VC, Kakolewski JW (1967) Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science 157:552–554
Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF (2011) Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav 98:570–574
Vivian JA, Miczek KA (1991) Ultrasounds during morphine withdrawal in rats. Psychopharmacology (Berl) 104:187–193
Vivian JA, Miczek KA (1993) Diazepam and gepirone selectively attenuate either 20–32 or 32–64 kHz ultrasonic vocalizations during aggressive encounters. Psychopharmacology (Berl) 112:66–73
Walker BM (2012) Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol (in press)
Walker BM, Koob GF (2007) The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 31:11–18
Walker BM, Koob GF (2008) Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33:643–652
Walker JR, Ahmed SH, Gracy KN, Koob GF (2000) Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res 854:85–92
Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF (2003) Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacol Biochem Behav 75:349–354
Walker BM, Rasmussen DD, Raskind MA, Koob GF (2008) Alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol 42:91–97
Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL (2010) Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol 44:487–493
Walker BM, Valdez GR, McLaughlin JP, Bakalkin G (2012) Targeting Dynorphin/Kappa Opioid Receptor Systems to Treat Alcohol Abuse and Dependence. Alcohol (in press)
Wright JS, Panksepp J (2011) Toward affective circuit-based preclinical models of depression: sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neurosci Biobehav Rev 35:1902–1915
Zhang Z, Morse AC, Koob GF, Schulteis G (2007) Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res 31:1811–1819
Acknowledgments
Support for this research was provided, in part, by R01AA020394-01 from the National Institute on Alcohol Abuse and Alcoholism, RGA 11-014 from the Hope for Depression Research Foundation, research grants from the WSU Alcohol and Drug Abuse Research Program awarded to BMW and DJR according to the State of Washington Initiative Measure No. 171, WSU Department of Psychology research grants awarded to ASP and DJR, and WSU Department of Pharmaceutical Science SURF awards to ASP, DJR, and LJN. The authors would like to thank the NIDA Drug Supply Program for their assistance with the study. The authors are particularly appreciative of the assistance provided by Dr. Jaak Panksepp and Paolo Iacobucci with the technicalities related to USV measurement and lively discussions related to affective states. None of the authors have any financial, personal or organizational conflicts of interest to report in relation to this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington.
Author information
Authors and Affiliations
Corresponding author
Additional information
Angela M. Williams, Daniel J. Reis, and Alexa S. Powell contributed equally to this study.
Rights and permissions
About this article
Cite this article
Williams, A.M., Reis, D.J., Powell, A.S. et al. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology 223, 75–88 (2012). https://doi.org/10.1007/s00213-012-2691-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2691-3