Abstract
Objective
To evaluate viability of isolated peripheral blood mononuclear cells (PBMC) and production of cytokines in vitro after stimulation as prognostic factors for survival in sepsis patients.
Design
Prospective study of the biological response of PBMC in the onset of severe sepsis.
Setting
Research laboratory of molecular biology and immunology and university hospital ICU, Faculty of Medicine, Trakia University.
Patients
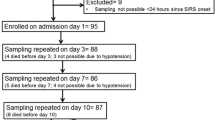
Twenty-three patients meeting the criteria for severe sepsis, and 14 control subjects.
Interventions
Isolated PBMC were stimulated in vitro with: C3-binding glycoprotein (C3bgp; 30 µg), lipopolysaccharide (30 µg), phytohemagglutinin (20 µg), pokeweed mitogen (30 µg), and dexamethasone (500 µg).
Measurements and results
We measured the levels of interleukins (IL) 6, 10, and 12 in culture supernatants. Stimulation with C3bgp and phytohemagglutinin led to significantly lower PBMC secretion of IL-6 in nonsurvivors than in survivors and healthy donors. Stimulation with C3bgp, lipopolysaccharide, and pokeweed mitogen considerably reduced IL-12 production in nonsurvivors. Stimulation with lipopolysaccharide and pokeweed mitogen caused immune cells in nonsurvivors to produce higher levels of IL-10 than in survivors. Survival of PBMC reduced viability for nonsurvivors’ PBMC, both spontaneously and as induced by lipopolysaccharide or pokeweed mitogen.
Conclusions
The viability of PBMC at the onset of sepsis and enhanced production of IL-12 and diminished production of IL-10 after stimulation with all stimuli used may be a favorable prognostic factor in sepsis.




Similar content being viewed by others
References
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA (2000) Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med 28:2591–2594
Geppert A, Steiner A, Zorn G, Delle-Karth G, Koreny M, Haumer M, Siostrzonek P, Huber K, Heinz G (2002) Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit Care Med 30:1987–1994
O’Brien LM, Abraham E (2004) Human models of endotoxemia and recombinant human activated protein C. Crit Care Med 32:S202–S208
Setrakian JC, Yee J, Christou NV (1994) Reduced tumor necrosis factor-α production in lipopolysaccharide-treated whole blood from patients in the intensive care unit. Arch Surg 129:187–192
Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg FW (1995) Downregulation of proinflamatory cytokine release in whole blood from septic patients. Blood 85:1341–1347
Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL (2001) Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J 15:879–892
Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drenou B (2002) Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 18:487–494
Abraham E, Andrews P, Antonelli M, Brochard L, Brun-Buisson C, Dobb G, Fagon JY, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pinsky M, Radermacher P, Ranieri M, Richard C, Tasker R, Vallet B (2004) Year in review in Intensive Care Medicine-2003 Part 1: Respiratory failure, infection and sepsis. Intensive Care Med 30:1017–1031
Muret J, Marie C, Fitting C, Payen D, Cavaillon JM (2000) Ex vivo T-lymphocyte derived cytokine production in SIRS patients is influenced by experimental procedures. Shock 13:169–174
Astiz M, Saha D, Lustbader D, Lin R, Rackow E (1996) Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med 128:597–600
Stanilova S, Karakolev Z, Dimov G, Dobreva Z, Slavov E (2003) Production of pro- and antiinflamatory cytokines upon stimulation of PBMC, isolated from patients in septic and postseptic stages. Immunol Lett 87:139
American College of Chest Physicians/Society of Critical Medicine Consensus Committee (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Vincent JL, Moreno R, Takala J (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure Intensive Care Med 22:707–710
Zhelev ZD, Stanilova SA, Carpenter BG (1994) Isolation, partial characterisation and complement inhibiting activity of a new glycoprotein from Cuscuta europea. Biochem Biophys Res Commun 202:186–194
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Little MC, Gawkrodger DJ, Macneil, S (1996) Chromium- and nickel-induced cytotoxicity in normal and transformed human keratinocytes: an investigation of pharmacological approaches to the prevention of Cr (VI)-induced cytotoxicity. Br J Dermatol 134:199–207
Zhang YY, Yu QH, You S, Sheng L (2004) Ginkgolides protect the cultured rat cortical cells against metabolic, excitotoxic and oxidative insults. J Health Sci 50:348–355
Mitov IG, Kropec A, Benzing A, Just H, Garota G, Galanos C, Freudenberg M (1997) Differential cytokine production in stimulated blood cultures from intensive care patients with bacterial infections. Infection 25:206–212
Stanilova S, Zhelev Z, Dobreva Z (2000) Preliminary study on the immunomodulatory effect of the C3 binding glycoprotein isolated from Cuscuta europea. Int J Immunopharmacol 22:15–24
Stanilova S, Dobreva Z, Zhelev Z (2001) Changes in serum levels of cytokines in mice injected with immunostimulator C3bgp isolated from Cuscuta europea. Int Immunopharmacol 1:1597–1604
Adamik B, Zimecki M, Wlaszczyk A, Kubler A (1997) Immunological status of septic and trauma patients. II. Proliferative response and production of interleukin 6 and tumor necrosis factor alpha by peripheral blood mononuclear cells from septic survivor, nonsurvivor and trauma patients: a correlation with the survival rate. Arch Immunol Ther Exp (Warsz) 45:277–284
Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG (2001) Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun 69:2099–2106
Perera PY, Vogel SN, Detore GR, Haziot A, Goyert SM (1997) CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol 158:4422–4429
Goebel A, Kavanagh E, Lyons A, Saporoschetz IB, Sobertg C, Lederer JA, Mannick JA, Rodrick ML (2000) Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg 231:253–261
Weighardt H, Heidecke CD, Emmanuilidis K, Maier S, Bartels H, Siewert JR, Holzmann B (2000) Sepsis after major visceral surgery is associated with sustained and interferon-gamma-resistant defect of monocytes cytokine production. Surgery 127:309–315
Weighardt H, Heidecke CD, Westerholt A, Emmanuilidis K, Maier S, Veit M, Gerauer K, Matevossian E, Ulm K, Siewert JR, Holzmann B (2002) Impaired monocyte IL-12 production before surgery as a predictive factor for the lethal outcome of postoperative sepsis. Ann Surg 235:560–567
Schwarz A, Stander S, Berneburg M, Bohm M, Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J, Schwarz T (2002) Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol 4:26–31
Napolitano LM, Campbell C (1995) Polymicrobial sepsis following trauma inhibits interleukin-10 secretion and lymphocyte proliferation. J Trauma 39:104–110
Gogos CA, Drosou E, Bassaris HP, Skoutelis A (2000) Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J Infect Dis 181:176–180
Monneret G, Finck ME, Venet F, Debard AL, Bohe J, Bienvenu J, Lepape A (2004) The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett 95:193–198
Wang SD, Huang KJ, Lin YS, Lei HY (1994) Sepsis-induced apoptosis of the thymocytes in mice. J Immunol 152:5014–5021
Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE (1997) Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-deficient mice. Crit Care Med 25:1298–1307
Oka M, Hirazawa K, Yamamoto K, Iizuka N, Hazama S, Suzuki T, Kobayashi N (1996) Induction of Fas-mediated apoptosis on circulating lymphocytes by surgical stress. Ann Surg 4:434–440
Acknowledgements
This study was supported by grant no. 1/2001 from the Fund for Scientific and Mobile Project, Faculty of Medicine, Trakia University, in Stara Zagora, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stanilova, S.A., Karakolev, Z.T., Dimov, G.S. et al. High interleukin 12 and low interleukin 10 production after in vitro stimulation detected in sepsis survivors. Intensive Care Med 31, 401–407 (2005). https://doi.org/10.1007/s00134-005-2575-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2575-7