Abstract
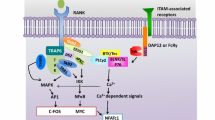
Recently a close relationship between the immune and skeletal systems or the interdisciplinary field called osteoimmunology has attracted much attention due to the observations that bone destruction is caused by an abnormal activation of the immune system in rheumatoid arthritis, and that mice lacking immunomodulatory molecules often exhibit an unexpected bone phenotype. Osteoclasts are cells of monocyte/macrophage origin that degrade the bone matrix. They are among the key players in the control of bone metabolism in health and disease. Receptor activator of NF-κB ligand (RANKL), a tumor necrosis factor (TNF) family cytokine, induces the differentiation of osteoclasts in the presence of macrophage-colony stimulating factor. RANKL activates TRAF6, c-Fos, and calcium signaling pathways, all of which are indispensable for the induction and activation of nuclear factor of activated T cells (NFAT) c1, the master transcription factor for osteoclastogenesis. The autoamplification of NFATc1 gene results in the efficient induction of osteoclast-specific genes. An AP-1 transcription factor complex containing c-Fos plays a crucial role in these processes, although results in conditional knockout mice show that Jun family members have a redundant role. The immunoreceptor tyrosine-based activation motif (ITAM) is an important signaling component for a number of receptors in the immune system including T-cell, B-cell, NK-cell, and Fc receptors, but its contribution to the skeletal system remains unclarified. In search for the calcium-mobilizing mechanism during osteoclastogenesis we determined that multiple immunoglobulinlike receptors associated with ITAM-harboring adaptors, Fc receptor common γ chain (FcRγ), and DNAX-activating protein (DAP) 12, are essential for osteoclastogenesis. In osteoclast precursor cells FcRγ-associated receptors include osteoclast-associated receptor and paired immunoglobulinlike receptor A, while triggering receptor expressed in myeloid cells 2 and signal-regulatory protein β1 preferentially associate with DAP12. In cooperation with RANKL these receptors activate phospholipase Cγ and calcium signaling essential for the induction of NFATc1 through ITAM phosphorylation. Thus we have established the importance of the ITAM-mediated costimulatory signals in RANKL-induced osteoclast differentiation, which is analogous to the role of costimulatory signals in the immune system. Here we summarize recent advances in the study of signaling mechanism of osteoclast differentiation in the context of osteoimmunology.






Similar content being viewed by others
Abbreviations
- AP :
-
Activator protein
- BMM :
-
Bone marrow-derived monocyte/macrophage precursor cell
- DAP :
-
DNAX-activating protein
- FcRγ :
-
Fc receptor common γ chain
- IFN :
-
Interferon
- ITAM :
-
Immunoreceptor tyrosine-based activation motif
- JNK :
-
Jun N-terminal kinase
- MAPK :
-
Mitogen-activated protein kinase
- M-CSF :
-
Macrophage-colony stimulating factor
- MITF :
-
Microphthalmia transcription factor
- NF :
-
Nuclear factor
- NFAT :
-
Nuclear factor of activated T cell
- OPG :
-
Osteoprotegerin
- OSCAR :
-
Osteoclast-associated receptor
- RANK :
-
Receptor activator of nuclear factor κB
- RANKL :
-
Receptor activator of nuclear factor κB ligand
- TNF :
-
Tumor necrosis factor
- TRAF :
-
TNF receptor-associated factor
- TREM :
-
Triggering receptor expressed in myeloid cells
References
Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S (2000) Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43:259–269
Karsenty G, Wagner EF (2002) Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2:389–406
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179
Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FSr, Frankel WN, Lee SY, Choi Y (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272:25190–25194
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Akinori T, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T (2000) T cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408:600–605
Arron JR, Choi Y (2000) Bone versus immune system. Nature 408:535–536
Kim N, Takami M, Rho J, Josien R, Choi Y (2002) A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med 195:201–209
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304–309
Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13:1015–1024
Theill LE, Boyle WJ, Penninger JM (2002) RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20:795–823
Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T (2002) RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature 416:744–749
Kim S, Koga T, Isobe M, Kern BE, Yokochi T, Chin YE, Karsenty G, Taniguchi T, Takayanagi H (2003) Stat1 functions as a cytoplasmic atttenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev 17:1979–1991
Feldmann M, Maini RN (2001) Anti-TNF α therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 19:163–196
Alliston T, Derynck R (2002) Interfering with bone remodelling. Nature 416:686–687
Baron R (2004) Arming the osteoclast. Nat Med 10:458–460
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357
Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123:2600–2602
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444
Lagasse E, Weissman IL (1997) Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell 89:1021–1031
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428:758–763
Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y (1998) The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J Biol Chem 273:28355–28359
Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4:353–362
Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J (2001) Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J 20:1271–1280
Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF (2000) Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet 24:184–187
Wagner EF, Karsenty G (2001) Genetic control of skeletal development. Curr Opin Genet Dev 11:527–532
Bohmann D, Bos TJ, Admon A, Nishimura T, Vogt PK, Tjian R (1987) Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science 238:1386–1392
Franza BR Jr, Rauscher FJ, 3rd, Josephs SF, Curran T (1988) The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science 239:1150–1153
Hai TW, Liu F, Allegretto EA, Karin M, Green MR (1988) A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev 2:1216–1226
Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev 11:3482–3496
Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266:443–448
Fleischmann A, Hafezi F, Elliott C, Reme CE, Ruther U, Wagner EF (2000) Fra-1 replaces c-Fos-dependent functions in mice. Genes Dev 14:2695–2700
David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF (2002) JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci 115:4317–4325
Kenner L, Hoebertz A, Beil T, Keon N, Karreth F, Eferl R, Scheuch H, Szremska A, Amling M, Schorpp-Kistner M, Angel P, Wagner EF (2004) Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J Cell Biol 164:613–623
Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, Kukita T, Yoshioka K, Rao A, Yoneda T (2004) Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest 114:475–484
Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ (1993) Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene 8:877–886
Brown PH, Chen TK, Birrer MJ (1994) Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene 9:791–799
Dong Z, Xu RH, Kim J, Zhan SN, Ma WY, Colburn NH, Kung H (1996) AP-1/jun is required for early Xenopus development and mediates mesoderm induction by fibroblast growth factor but not by activin. J Biol Chem 271:9942–9946
Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL (1997) Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386:81–84
Weilbaecher KN, Motyckova G, Huber WE, Takemoto CM, Hemesath TJ, Xu Y, Hershey CL, Dowland NR, Wells AG, Fisher DE (2001) Linkage of M-CSF signaling to Mitf, TFE3, and the osteoclast defect in Mitfmi/mi mice. Mol Cell 8:749–758
McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, Jordan SA, Jackson IJ, Korsmeyer SJ, Golub TR, Fisher DE (2002) Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109:707–718
So H, Rho J, Jeong D, Park R, Fisher DE, Ostrowski MC, Choi Y, Kim N (2003) Microphthalmia transcription factor and PU.1 synergistically induce the leukocyte receptor osteoclast-associated receptor gene expression. J Biol Chem 278:24209–24216
Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, Hisatake K, Nogi Y (2004) Essential role of p38 MAP kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem 279:969–979
Shirakawa F, Chedid M, Suttles J, Pollok BA, Mizel SB (1989) Interleukin 1 and cyclic AMP induce κ immunoglobulin light-chain expression via activation of an NF-κB-like DNA-binding protein. Mol Cell Biol 9:959–964
Muegge K, Williams TM, Kant J, Karin M, Chiu R, Schmidt A, Siebenlist U, Young HA, Durum SK (1989) Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science 246:249–251
Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K (1999) The kinase TAK1 can activate the NIK-I κB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252–256
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling for terminal differentiation of osteoclasts. Dev Cell 3:889–901
Rao A, Luo C, Hogan PG (1997) Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15:707–747
Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR (1988) Identification of a putative regulator of early T cell activation genes. Science 241:202–205
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21
Crabtree GR, Olson EN (2002) NFAT signaling: choreographing the social lives of cells. Cell 109 [Suppl]:S67–S79
Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277:41147–41156
Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279:26475–26480
Munaut C, Salonurmi T, Kontusaari S, Reponen P, Morita T, Foidart JM, Tryggvason K (1999) Murine matrix metalloproteinase 9 gene. 5’-upstream region contains cis-acting elements for expression in osteoclasts and migrating keratinocytes in transgenic mice. J Biol Chem 274:5588–5596
Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, Kanazawa K, Tan-Takeuchi K, Iwasaki K, Yokoyama WM, Kudo A, Fujiwara M, Asou H, Takai T (2003) Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest 111:323–332
Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC (2004) The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA 101:6158–6163
Cerwenka A, Lanier LL (2001) Natural killer cells, viruses and cancer. Nat Rev Immunol 1:41–49
Takai T (2002) Roles of Fc receptors in autoimmunity. Nat Rev Immunol 2:580–592
Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP (2003) High dose M-CSF partially rescues the Dap12-/- osteoclast phenotype. J Cell Biochem 90:871–883
Aoki K, Didomenico E, Sims NA, Mukhopadhyay K, Neff L, Houghton A, Amling M, Levy JB, Horne WC, Baron R (1999) The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: increased resorption and osteopenia in mev/mev mutant mice. Bone 25:261–267
Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ, Brodt MD, Helgason CD, Kalesnikoff J, Rauh MJ, Humphries RK, Krystal G, Teitelbaum SL, Ross FP (2002) SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med 8:943–949
Hayashi S, Tsuneto M, Yamada T, Nose M, Yoshino M, Shultz LD, Yamazaki H (2004) Lipopolysaccharide-induced osteoclastogenesis in Src homology 2-domain phosphatase-1-deficient viable motheaten mice. Endocrinology 145:2721–2729
Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71:656–662
Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L (2003) DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med 198:669–675
Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M (2003) Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med 198:645–651
Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S (2002) Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med 347:175–184
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS (1998) osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268
Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, Anderson DM (2000) Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet 24:45–48
Whyte MP, Hughes AE (2002) Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J Bone Miner Res 17:26–29
Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, Holmes GB, Dunstan CR, DePaoli AM (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19:1059–1066
Acknowledgements
I appreciate T. Taniguchi, T. Takai, M. Inui, T. Koga and S. Kim for their great contribution to the publications, on which this work is based. I also thank K. Matsuo, T. Nakajima, A. Suematsu, K. Sato, M. Asagiri and Y. Kim for critical reading of the manuscript and discussion. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and JSPS, the PRESTO program of JST, grants for the 21st century COE program, Health Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan, and grants from the Mitsubishi Foundation, The Kato Trust for Nambyo Research and Takeda Science foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takayanagi, H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83, 170–179 (2005). https://doi.org/10.1007/s00109-004-0612-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-004-0612-6