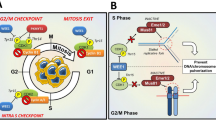
Abstract
Y‑box binding protein‑1 (YB-1) is a multifunctional protein that is highly expressed in human solid tumors of various entities. Several cellular processes, e.g. cell cycle progression, cancer stemness and DNA damage signaling that are involved in the response to chemoradiotherapy (CRT) are tightly governed by YB‑1. KRAS gene with about 30% mutations in all cancers, is considered the most commonly mutated oncogene in human cancers. Accumulating evidence indicates that oncogenic KRAS mediates CRT resistance. AKT and p90 ribosomal S6 kinase are downstream of KRAS and are the major kinases that stimulate YB‑1 phosphorylation. Thus, there is a close link between the KRAS mutation status and YB‑1 activity. In this review paper, we highlight the importance of the KRAS/YB‑1 cascade in the response of KRAS-mutated solid tumors to CRT. Likewise, the opportunities to interfere with this pathway to improve CRT outcome are discussed in light of the current literature.



Similar content being viewed by others
References
Dolfini D, Mantovani R (2013) Targeting the Y/CCAAT box in cancer: YB‑1 (YBX1) or NF-Y? Cell Death Differ 20(5):676–685
Lindquist JA, Mertens PR (2018) Cold shock proteins: from cellular mechanisms to pathophysiology and disease. Cell Commun Signal 16(1):63
Zhan Y et al (2022) YB1 associates with oncogenetic roles and poor prognosis in nasopharyngeal carcinoma. Sci Rep 12(1):3699
Yahata H et al (2002) Increased nuclear localization of transcription factor YB‑1 in acquired cisplatin-resistant ovarian cancer. J Cancer Res Clin Oncol 128(11):621–626
Nishio S et al (2014) Nuclear Y‑box-binding protein‑1 is a poor prognostic marker and related to epidermal growth factor receptor in uterine cervical cancer. Gynecol Oncol 132(3):703–708
Dahl E et al (2009) Nuclear detection of Y‑boxprotein‑1 (YB-1) closely associates with progesterone receptor negativity and is a strong adverse survival factor in human breast cancer. BMC Cancer 9(1):410
Fushimi F et al (2013) Peroxiredoxins, thioredoxin, and Y‑box-binding protein‑1 are involved in the pathogenesis and progression of dialysis-associated renal cell carcinoma. Virchows Arch 463(4):553–562
Sheridan CM et al (2015) YB‑1 and MTA1 protein levels and not DNA or mRNA alterations predict for prostate cancer recurrence. Oncotarget 6(10):7470–7480
Shibahara K et al (2001) Nuclear expression of the Y‑box binding protein, YB‑1, as a novel marker of disease progression in non-small cell lung cancer1. Clin Cancer Res 7(10):3151–3155
Guo T et al (2017) YB‑1 regulates tumor growth by promoting MACC1/c-Met pathway in human lung adenocarcinoma. Oncotarget 8(29):48110–48125
Sinnberg T et al (2012) MAPK and PI3K/AKT mediated YB‑1 activation promotes melanoma cell proliferation which is counteracted by an autoregulatory loop. Exp Dermatol 21(4):265–270
Song YH et al (2014) Twist1 and Y‑box-binding protein‑1 are potential prognostic factors in bladder cancer. Urol Oncol 32(1):31.e1–31.e7
Chao H‑M et al (2016) Y‑box binding protein‑1 promotes hepatocellular carcinoma-initiating cell progression and tumorigenesis via Wnt/β-catenin pathway. Oncotarget 8(2):2604–2616
Yan X et al (2014) High expression of Y‑box-binding protein 1 is associated with local recurrence and predicts poor outcome in patients with colorectal cancer. Int J Clin Exp Pathol 7(12):8715–8723
Zhang Y et al (2012) Overexpression of Y‑box binding protein‑1 in cervical cancer and its association with the pathological response rate to chemoradiotherapy. Med Oncol 29(3):1992–1997
Mylona E et al (2014) Y‑box-binding protein 1 (YB1) in breast carcinomas: relation to aggressive tumor phenotype and identification of patients at high risk for relapse. Eur J Surg Oncol 40(3):289–296
Shibahara K et al (2004) Targeted disruption of one allele of the Y‑box binding protein‑1 (YB-1) gene in mouse embryonic stem cells and increased sensitivity to cisplatin and mitomycin C. Cancer Sci 95(4):348–353
Chatterjee M et al (2008) The Y‑box binding protein YB‑1 is associated with progressive disease and mediates survival and drug resistance in multiple myeloma. Blood 111(7):3714–3722
Kim ER et al (2013) The proteolytic YB‑1 fragment interacts with DNA repair machinery and enhances survival during DNA damaging stress. Cell Cycle 12(24):3791–3803
Johnson TG et al (2019) Why be one protein when you can affect many? The multiple roles of YB‑1 in lung cancer and mesothelioma. Front Cell Dev Biol 7:221
Kohno Y et al (2006) Expression of Y‑box-binding protein dbpC/contrin, a potentially new cancer/testis antigen. Br J Cancer 94(5):710–716
Lyabin DN et al (2020) YB‑3 substitutes YB‑1 in global mRNA binding. RNA Biol 17(4):487–499
Wang GR et al (2009) Upregulation of human DNA binding protein A (dbpA) in gastric cancer cells. Acta Pharmacol Sin 30(10):1436–1442
Liu RT et al (2016) RNAi-mediated downregulation of DNA binding protein A inhibits tumorigenesis in colorectal cancer. Int J Mol Med 38(3):703–712
Yasen M et al (2005) The up-regulation of Y‑box binding proteins (DNA binding protein A and Y‑box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin Cancer Res 11(20):7354–7361
Hayashi J et al (2002) Somatic mutation and SNP in the promoter of dbpA and human hepatocarcinogenesis. Int J Oncol 21(4):847–850
Nakatsura T et al (2001) Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun 281(4):936–944
Hohlfeld R et al (2018) Crosstalk between Akt signaling and cold shock proteins in mediating invasive cell phenotypes. Oncotarget 9(27):19039–19049
Wang W et al (2016) Antimicrobial peptide LL-37 promotes the proliferation and invasion of skin squamous cell carcinoma by upregulating DNA-binding protein A. Oncol Lett 12(3):1745–1752
Tong C et al (2020) Knockdown of DNA-binding protein A enhances the chemotherapy sensitivity of colorectal cancer via suppressing the Wnt/β-catenin/Chk1 pathway. Cell Biol Int 44(10):2075–2085
Yang X‑J et al (2019) Crystal structure of a Y-box binding protein 1 (YB-1)–RNA complex reveals key features and residues interacting with RNA. J Biol Chem 294(28):10998–11010
Zhang J et al (2020) Structural basis of DNA binding to human YB‑1 cold shock domain regulated by phosphorylation. Nucleic Acids Res 48(16):9361–9371
Matsumoto K, Wolffe AP (1998) Gene regulation by Y‑box proteins: coupling control of transcription and translation. Trends Cell Biol 8(8):318–323
Lyabin DN, Eliseeva IA, Ovchinnikov LP (2014) YB‑1 protein: functions and regulation. Wiley Interdiscip Rev RNA 5(1):95–110
Evdokimova VM et al (1998) The major core protein of messenger ribonucleoprotein particles (p50) promotes initiation of protein biosynthesis in vitro. J Biol Chem 273(6):3574–3581
Evdokimova VM et al (1995) The major protein of messenger ribonucleoprotein particles in somatic cells is a member of the Y‑box binding transcription factor family. J Biol Chem 270(7):3186–3192
Minich WB, Maidebura IP, Ovchinnikov LP (1993) Purification and characterization of the major 50-kDa repressor protein from cytoplasmic mRNP of rabbit reticulocytes. Eur J Biochem 212(3):633–638
Hamon L, Budkina K, Pastré D (2022) YB‑1 structure/function relationship in the packaging of mRNPs and consequences for translation regulation and stress granule assembly in cells. Biochemistry 87(1):S20–S31
van Roeyen CRC et al (2013) Cold shock Y‑box protein‑1 proteolysis autoregulates its transcriptional activities. Cell Commun Signal 11(1):63
Hamon L, Budkina K, Pastre D (2022) YB‑1 structure/function relationship in the packaging of mRNPs and consequences for translation regulation and stress granule assembly in cells. Biochemistry (Mosc) 87(1):S20–S93
Perner F et al (2022) YBX1 mediates translation of oncogenic transcripts to control cell competition in AML. Leukemia 36(2):426–437
El-Naggar AM et al (2015) Translational activation of HIF1α by YB‑1 promotes sarcoma metastasis. Cancer Cell 27(5):682–697
Evdokimova V et al (2009) Translational activation of snail1 and other developmentally regulated transcription factors by YB‑1 promotes an epithelial-mesenchymal transition. Cancer Cell 15(5):402–415
Evdokimova V et al (2001) The major mRNA-associated protein YB‑1 is a potent 5′ cap-dependent mRNA stabilizer. EMBO J 20(19):5491–5502
Evdokimova VM, Ovchinnikov LP (1999) Translational regulation by Y‑box transcription factor: involvement of the major mRNA-associated protein, p50. Int J Biochem Cell Biol 31(1):139–149
Minich WB, Ovchinnikov LP (1992) Role of cytoplasmic mRNP proteins in translation. Biochimie 74(5):477–483
Nekrasov MP et al (2003) The mRNA-binding protein YB‑1 (p50) prevents association of the eukaryotic initiation factor eIF4G with mRNA and inhibits protein synthesis at the initiation stage. J Biol Chem 278(16):13936–13943
McKernan CM et al (2022) ABL kinases regulate translation in HER2+ cells through Y‑box-binding protein 1 to facilitate colonization of the brain. Cell Rep 40(9):111268
Kosnopfel C et al (2018) YB‑1 expression and phosphorylation regulate tumorigenicity and invasiveness in melanoma by influencing EMT. Mol Cancer Res 16(7):1149–1160
Somasekharan SP et al (2015) YB‑1 regulates stress granule formation and tumor progression by translationally activating G3BP1. J Cell Biol 208(7):913–929
Chu P‑C et al (2018) Mutant KRAS promotes liver metastasis of colorectal cancer, in part, by upregulating the MEK-Sp1-DNMT1-miR-137-YB-1-IGF-IR signaling pathway. Oncogene 37(25):3440–3455
Koike K et al (1997) Nuclear translocation of the Y‑box binding protein by ultraviolet irradiation. FEBS Lett 417(3):390–394
Rauen T et al (2016) Cold shock protein YB‑1 is involved in hypoxia-dependent gene transcription. Biochem Biophys Res Commun 478(2):982–987
Woolley AG et al (2011) Prognostic association of YB‑1 expression in breast cancers: a matter of antibody. PLoS One 6(6):e20603
Yokoyama H et al (2003) Regulation of YB‑1 gene expression by GATA transcription factors. Biochem Biophys Res Commun 303(1):140–145
Uramoto H et al (2002) p73 Interacts with c‑Myc to regulate Y‑box-binding protein‑1 expression. J Biol Chem 277(35):31694–31702
Bommert KS et al (2013) The feed-forward loop between YB‑1 and MYC is essential for multiple myeloma cell survival. Leukemia 27(2):441–450
Shiota M et al (2008) Twist promotes tumor cell growth through YB‑1 expression. Cancer Res 68(1):98–105
Kobayashi S et al (2015) YB‑1 gene expression is kept constant during myocyte differentiation through replacement of different transcription factors and then falls gradually under the control of neural activity. Int J Biochem Cell Biol 68:1–8
Skabkina OV et al (2005) YB‑1 autoregulates translation of its own mRNA at or prior to the step of 40S ribosomal subunit joining. Mol Cell Biol 25(8):3317–3323
Skabkina OV et al (2003) Poly(A)-binding protein positively affects YB‑1 mRNA translation through specific interaction with YB‑1 mRNA. J Biol Chem 278(20):18191–18198
Lyabin DN, Eliseeva IA, Ovchinnikov LP (2012) YB‑1 synthesis is regulated by mTOR signaling pathway. PLoS One 7(12):e52527
Lu J et al (2017) YB‑1 expression promotes pancreatic cancer metastasis that is inhibited by microRNA-216a. Exp Cell Res 359(2):319–326
Zhao X et al (2020) Circ-SAR1A promotes renal cell carcinoma progression through miR-382/YBX1 axis. Cancer Manag Res 12:7353–7361
Liu SL, Sui YF, Lin MZ (2016) MiR-375 is epigenetically downregulated due to promoter methylation and modulates multi-drug resistance in breast cancer cells via targeting YBX1. Eur Rev Med Pharmacol Sci 20(15):3223–3229
Stratford AL et al (2007) Epidermal growth factor receptor (EGFR) is transcriptionally induced by the Y‑box binding protein‑1 (YB-1) and can be inhibited with Iressa in basal-like breast cancer, providing a potential target for therapy. Breast Cancer Res 9(5):R61
Wu J et al (2006) Disruption of the Y‑box binding protein‑1 results in suppression of the epidermal growth factor receptor and HER‑2. Cancer Res 66(9):4872–4879
Bader AG, Vogt PK (2008) Phosphorylation by Akt disables the anti-oncogenic activity of YB‑1. Oncogene 27(8):1179–1182
Gieseler-Halbach S et al (2017) RSK-mediated nuclear accumulation of the cold-shock Y‑box protein‑1 controls proliferation of T cells and T‑ALL blasts. Cell Death Differ 24(2):371–383
Sutherland BW et al (2005) Akt phosphorylates the Y‑box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene 24(26):4281–4292
Tiwari A et al (2018) Stress-induced phosphorylation of nuclear YB‑1 depends on nuclear trafficking of p90 ribosomal S6 kinase. Int J Mol Sci 19(8):2441. https://doi.org/10.3390/ijms19082441
Sogorina EM et al (2022) YB‑1 phosphorylation at serine 209 inhibits its nuclear translocation. Int J Mol Sci 23(1):428
Mehta S et al (2020) Dephosphorylation of YB‑1 is required for nuclear localisation during G(2) phase of the cell cycle. Cancers (Basel) 12(2):315
Jayavelu AK et al (2020) Splicing factor YBX1 mediates persistence of JAK2-mutated neoplasms. Nature 588(7836):157–163
Nöthen T et al (2023) DNA-dependent protein kinase mediates YB‑1 (Y-box binding protein)-induced double strand break repair. ATVB 43(2):300–311
Wang J et al (2016) Therapeutic nuclear shuttling of YB‑1 reduces renal damage and fibrosis. Kidney Int 90(6):1226–1237
Kretov DA et al (2020) Inhibition of transcription induces phosphorylation of YB‑1 at Ser102 and its accumulation in the nucleus. Cells 9(1):104
Prabhu L et al (2015) Critical role of phosphorylation of serine 165 of YBX1 on the activation of NF-κB in colon cancer. Oncotarget 6(30):29396–29412
Martin M et al (2017) Novel serine 176 phosphorylation of YBX1 activates NF-kappaB in colon cancer. J Biol Chem 292(8):3433–3444
Mehta S et al (2020) Critical role for cold shock protein YB‑1 in cytokinesis. Cancers (Basel) 12(9):2473. https://doi.org/10.3390/cancers12092473
El-Naggar AM et al (2019) Class I HDAC inhibitors enhance YB‑1 acetylation and oxidative stress to block sarcoma metastasis. EMBO Rep 20(12):e48375
Frye BC et al (2009) Y‑box protein‑1 is actively secreted through a non-classical pathway and acts as an extracellular mitogen. EMBO Rep 10(7):783–789
Kim W et al (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 44(2):325–340
Palicharla VR, Maddika S (2015) HACE1 mediated K27 ubiquitin linkage leads to YB‑1 protein secretion. Cell Signal 27(12):2355–2362
Hartley AV et al (2020) PRMT5-mediated methylation of YBX1 regulates NF-κB activity in colorectal cancer. Sci Rep 10(1):15934
Geiss-Friedlander R, Melchior F (2007) Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8(12):947–956
Mai RT et al (2022) Sumoylation participates in the regulation of YB-1-mediated mismatch repair deficiency and alkylator tolerance. Am J Cancer Res 12(12):5462–5483
Chang YW et al (2014) YB‑1 disrupts mismatch repair complex formation, interferes with MutSα recruitment on mismatch and inhibits mismatch repair through interacting with PCNA. Oncogene 33(43):5065–5077
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418(6901):935–941
Pagano C et al (2017) The tumor-associated YB‑1 protein: new player in the circadian control of cell proliferation. Oncotarget 8(4):6193–6205
Liu Q et al (2016) Hyper-O-GlcNAcylation of YB‑1 affects Ser102 phosphorylation and promotes cell proliferation in hepatocellular carcinoma. Exp Cell Res 349(2):230–238
Fujiwara-Okada Y et al (2013) Y‑box binding protein‑1 regulates cell proliferation and is associated with clinical outcomes of osteosarcoma. Br J Cancer 108(4):836–847
Lasham A et al (2012) YB‑1, the E2F pathway, and regulation of tumor cell growth. J Natl Cancer Inst 104(2):133–146
Jurchott K et al (2003) YB‑1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J Biol Chem 278(30):27988–27996
Tiwari A et al (2020) Blocking Y‑box binding protein‑1 through simultaneous targeting of PI3K and MAPK in triple negative breast cancers. Cancers (Basel) 12(10):2795. https://doi.org/10.3390/cancers12102795
Gaudreault I, Guay D, Lebel M (2004) YB‑1 promotes strand separation in vitro of duplex DNA containing either mispaired bases or cisplatin modifications, exhibits endonucleolytic activities and binds several DNA repair proteins. Nucleic Acids Res 32(1):316–327
Toulany M et al (2011) Impact of oncogenic K‑RAS on YB‑1 phosphorylation induced by ionizing radiation. Breast Cancer Res 13(2):R28
Lettau K, Zips D, Toulany M (2021) Simultaneous targeting of RSK and AKT efficiently inhibits YB-1-mediated repair of ionizing radiation-induced DNA double-strand breaks in breast cancer cells. Int J Radiat Oncol Biol Phys 109(2):567–580
Khozooei S et al (2022) Fisetin induces DNA double-strand break and interferes with the repair of radiation-induced damage to radiosensitize triple negative breast cancer cells. J Exp Clin Cancer Res 41(1):256
Ise T et al (1999) Transcription factor Y‑box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res 59(2):342–346
Hasegawa SL et al (1991) DNA binding properties of YB‑1 and dbpA: binding to double-stranded, single-stranded, and abasic site containing DNAs. Nucleic Acids Res 19(18):4915–4920
Naumenko KN et al (2020) Regulation of poly(ADP-ribose) polymerase 1 activity by Y‑box-binding protein 1. Biomolecules 10(9):1325
Alemasova EE et al (2018) The multifunctional protein YB‑1 potentiates PARP1 activity and decreases the efficiency of PARP1 inhibitors. Oncotarget 9(34):23349–23365
Lettau K et al (2021) Targeting the Y‑box binding protein‑1 axis to overcome radiochemotherapy resistance in solid tumors. Int J Radiat Oncol Biol Phys 111(4):1072–1087
Kosnopfel C, Sinnberg T, Schittek B (2014) Y‑box binding protein 1—a prognostic marker and target in tumour therapy. Eur J Cell Biol 93(1):61–70
Toulany M (2023) Targeting K‑Ras-mediated DNA damage response in radiation oncology: current status, challenges and future perspectives. Clin Transl Radiat Oncol 38:6–14
Shinkai K et al (2016) Nuclear expression of Y‑box binding protein‑1 is associated with poor prognosis in patients with pancreatic cancer and its knockdown inhibits tumor growth and metastasis in mice tumor models. Int J Cancer 139(2):433–445
Shiraiwa S et al (2016) Nuclear Y‑box-binding protein‑1 expression predicts poor clinical outcome in stage III colorectal cancer. Anticancer Res 36(7):3781–3788
Ardito F et al (2014) Strong YB‑1 expression predicts liver recurrence following resection for colorectal metastases. J Gastrointest Surg 18(11):1987–1993
Zhang Y et al (2015) The expression level and prognostic value of Y‑box binding protein‑1 in rectal cancer. PLoS One 10(3):e119385
Nagasu S et al (2019) Yboxbinding protein 1 inhibits apoptosis and upregulates EGFR in colon cancer. Oncol Rep 41(5):2889–2896
Kashihara M et al (2009) Nuclear Y‑box binding protein‑1, a predictive marker of prognosis, is correlated with expression of HER2/ErbB2 and HER3/ErbB3 in non-small cell lung cancer. J Thorac Oncol 4(9):1066–1074
Gessner C et al (2004) Nuclear YB‑1 expression as a negative prognostic marker in nonsmall cell lung cancer. Eur Respir J 23(1):14–19
Jiang L et al (2017) Positive expression of Y‑box binding protein 1 and prognosis in non-small cell lung cancer: a meta-analysis. Oncotarget 8(33):55613–55621
Prior IA, Hood FE, Hartley JL (2020) The frequency of Ras mutations in cancer. Cancer Res 80(14):2969–2974
Raso E (2020) Splice variants of RAS-translational significance. Cancer Metastasis Rev 39(4):1039–1049
Nuevo-Tapioles C, Philips MR (2022) The role of KRAS splice variants in cancer biology. Front Cell Dev Biol 10:1033348
Schlichting I et al (1990) Time-resolved X‑ray crystallographic study of the conformational change in Ha-Ras p21 protein on GTP hydrolysis. Nature 345(6273):309–315
Milburn MV et al (1990) Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science 247(4945):939–945
Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294(5545):1299–1304
Bos JL, Rehmann H, Wittinghofer A (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129(5):865–877
Saraste M, Sibbald PR, Wittinghofer A (1990) The P‑loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci 15(11):430–434
Willumsen BM et al (1984) The p21 ras C‑terminus is required for transformation and membrane association. Nature 310(5978):583–586
Gideon P et al (1992) Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: the C‑terminal domain of GAP is not sufficient for full activity. Mol Cell Biol 12(5):2050–2056
Hobbs GA, Der CJ, Rossman KL (2016) RAS isoforms and mutations in cancer at a glance. J Cell Sci 129(7):1287–1292
Cammarata MB et al (2016) Impact of G12 mutations on the structure of K‑Ras probed by ultraviolet photodissociation mass spectrometry. J Am Chem Soc 138(40):13187–13196
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D (2011) RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 11(11):761–774
Zhang XF et al (1993) Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c‑Raf‑1. Nature 364(6435):308–313
Moodie SA et al (1993) Complexes of Ras.GTP with Raf‑1 and mitogen-activated protein kinase kinase. Science 260(5114):1658–1661
Rodriguez-Viciana P et al (1997) Role of phosphoinositide 3‑OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89(3):457–467
White MA et al (1996) A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem 271(28):16439–16442
Eser S et al (2014) Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer 111(5):817–822
Jones RP et al (2017) Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer 116(7):923–929
Imamura Y et al (2012) Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer Res 18(17):4753–4763
Buscail L, Bournet B, Cordelier P (2020) Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol 17(3):153–168
Goulding RE et al (2020) KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: a systematic literature review and meta-analysis. Cancer Treat Res Commun 24:100200
Gurtner K et al (2020) Radioresistance of KRAS/TP53-mutated lung cancer can be overcome by radiation dose escalation or EGFR tyrosine kinase inhibition in vivo. Int J Cancer 147(2):472–477
Duldulao MP et al (2013) Mutations in specific codons of the KRAS oncogene are associated with variable resistance to neoadjuvant chemoradiation therapy in patients with rectal adenocarcinoma. Ann Surg Oncol 20(7):2166–2171
Mak RH et al (2015) Outcomes by tumor histology and KRAS mutation status after lung stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Clin Lung Cancer 16(1):24–32
Metro G et al (2014) Clinical outcome with platinum-based chemotherapy in patients with advanced nonsquamous EGFR wild-type non-small-cell lung cancer segregated according to KRAS mutation status. Clin Lung Cancer 15(1):86–92
Lievre A et al (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66(8):3992–3995
Benvenuti S et al (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67(6):2643–2648
Forster T et al (2020) Cetuximab in pancreatic cancer therapy: a systematic review and meta-analysis. Oncology 98(1):53–60
Ridouane Y et al (2017) Targeted first-line therapies for advanced colorectal cancer: a Bayesian meta-analysis. Oncotarget 8(39):66458–66466
Smith MJ, Neel BG, Ikura M (2013) NMR-based functional profiling of RASopathies and oncogenic RAS mutations. Proc Natl Acad Sci U S A 110(12):4574–4579
Lu S et al (2016) The structural basis of oncogenic mutations G12, G13 and Q61 in small GTPase K‑Ras4B. Sci Rep 6:21949
Khrenova MG et al (2014) Modeling the role of G12V and G13V Ras mutations in the Ras-GAP-catalyzed hydrolysis reaction of guanosine triphosphate. Biochemistry 53(45):7093–7099
Chen CC et al (2013) Computational analysis of KRAS mutations: implications for different effects on the KRAS p.G12D and p.G13D mutations. PLoS One 8(2):e55793
Hunter JC et al (2015) Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol Cancer Res 13(9):1325–1335
Munoz-Maldonado C, Zimmer Y, Medova M (2019) A comparative analysis of individual RAS mutations in cancer biology. Front Oncol 9:1088
Buhrman G, Wink G, Mattos C (2007) Transformation efficiency of RasQ61 mutants linked to structural features of the switch regions in the presence of Raf. Structure 15(12):1618–1629
Haigis KM (2017) KRAS alleles: the devil is in the detail. Trends Cancer 3(10):686–697
Ihle NT et al (2012) Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst 104(3):228–239
Hammond DE et al (2015) Differential reprogramming of isogenic colorectal cancer cells by distinct activating KRAS mutations. J Proteome Res 14(3):1535–1546
Garassino MC et al (2011) Different types of K‑Ras mutations could affect drug sensitivity and tumour behaviour in non-small-cell lung cancer. Ann Oncol 22(1):235–237
Cook JH et al (2021) The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat Commun 12(1):1808
Jia Y et al (2017) Characterization of distinct types of KRAS mutation and its impact on first-line platinum-based chemotherapy in Chinese patients with advanced non-small cell lung cancer. Oncol Lett 14(6):6525–6532
Nadal E et al (2014) KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol 9(10):1513–1522
Dai M et al (2022) Prognostic value of KRAS subtype in patients with PDAC undergoing radical resection. Front Oncol 12:1074538
Park HE et al (2021) Tumor microenvironment-adjusted prognostic implications of the KRAS mutation subtype in patients with stage III colorectal cancer treated with adjuvant FOLFOX. Sci Rep 11(1):14609
Chida K et al (2021) The prognostic impact of KRAS G12C mutation in patients with metastatic colorectal cancer: a multicenter retrospective observational study. Oncologist 26(10):845–853
Rabara D et al (2019) KRAS G13D sensitivity to neurofibromin-mediated GTP hydrolysis. Proc Natl Acad Sci USA 116(44):22122–22131
Stratford AL et al (2008) Y‑box binding protein‑1 serine 102 is a downstream target of p90 ribosomal S6 kinase in basal-like breast cancer cells. Breast Cancer Res 10(6):R99
Evdokimova V et al (2006) Akt-mediated YB‑1 phosphorylation activates translation of silent mRNA species. Mol Cell Biol 26(1):277–292
Lee CS et al (2019) MAP kinase and autophagy pathways cooperate to maintain RAS mutant cancer cell survival. Proc Natl Acad Sci U S A 116(10):4508–4517
Takacs T et al (2020) The effects of mutant Ras proteins on the cell signalome. Cancer Metastasis Rev 39(4):1051–1065
Higuchi M et al (2008) Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol 10(11):1356–1364
Beeser A et al (2005) Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem 280(44):36609–36615
Lu H et al (2017) PAK signalling drives acquired drug resistance to MAPK inhibitors in BRAF-mutant melanomas. Nature 550(7674):133–136
Zang M, Hayne C, Luo Z (2002) Interaction between active Pak1 and Raf‑1 is necessary for phosphorylation and activation of Raf‑1. J Biol Chem 277(6):4395–4405
Eblen ST et al (2002) Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol 22(17):6023–6033
Park ER, Eblen ST, Catling AD (2007) MEK1 activation by PAK: a novel mechanism. Cell Signal 19(7):1488–1496
Wang Z et al (2013) p21-activated kinase 1 (PAK1) can promote ERK activation in a kinase-independent manner. J Biol Chem 288(27):20093–20099
Tang Y et al (2000) The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem 275(13):9106–9109
Zhou GL et al (2003) Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol Cell Biol 23(22):8058–8069
King CC et al (2000) p21-activated kinase (PAK1) is phosphorylated and activated by 3‑phosphoinositide-dependent kinase‑1 (PDK1). J Biol Chem 275(52):41201–41209
Ebi H et al (2013) PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P‑Rex1. Proc Natl Acad Sci U S A 110(52):21124–21129
Thillai K et al (2017) Deciphering the link between PI3K and PAK: an opportunity to target key pathways in pancreatic cancer? Oncotarget 8(8):14173–14191
McCarty SK et al (2014) BRAF activates and physically interacts with PAK to regulate cell motility. Endocr Relat Cancer 21(6):865–877
Linardou H et al (2008) Assessment of somatic k‑RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 9(10):962–972
Normanno N et al (2009) Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 6(9):519–527
Knickelbein K, Zhang L (2015) Mutant KRAS as a critical determinant of the therapeutic response of colorectal cancer. Genes Dis 2(1):4–12
Eberhard DA et al (2005) Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23(25):5900–5909
Sumi S et al (1992) Inhibition of pancreatic adenocarcinoma cell growth by lovastatin. Gastroenterology 103(3):982–989
Kohl NE et al (1994) Protein farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci U S A 91(19):9141–9145
Sun J et al (1995) Ras CAAX peptidomimetic FTI 276 selectively blocks tumor growth in nude mice of a human lung carcinoma with K‑Ras mutation and p53 deletion. Cancer Res 55(19):4243–4247
Santillo M et al (1996) Inhibitors of Ras farnesylation revert the increased resistance to oxidative stress in K‑Ras transformed NIH 3T3 cells. Biochem Biophys Res Commun 229(3):739–745
Hunt JT et al (2000) Discovery of (R)-7-cyano‑2,3,4, 5‑tetrahydro-1-(1H-imidazol-4-ylmethyl)-3-(phenylmethyl)-4-(2-thienylsulfonyl)-1H‑1,4‑benzodiazepine (BMS-214662), a farnesyltransferase inhibitor with potent preclinical antitumor activity. J Med Chem 43(20):3587–3595
Macdonald JS et al (2005) A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs 23(5):485–487
Rao S et al (2004) Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol 22(19):3950–3957
Cohen SJ et al (2003) Phase II and pharmacodynamic study of the farnesyltransferase inhibitor R115777 as initial therapy in patients with metastatic pancreatic adenocarcinoma. J Clin Oncol 21(7):1301–1306
Adjei AA et al (2003) Phase II study of the farnesyl transferase inhibitor R115777 in patients with advanced non-small-cell lung cancer. J Clin Oncol 21(9):1760–1766
Whyte DB et al (1997) K‑ and N‑Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem 272(22):14459–14464
Lerner EC et al (1997) Inhibition of the prenylation of K‑Ras, but not H‑ or N‑Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene 15(11):1283–1288
Martin NE et al (2004) A phase I trial of the dual farnesyltransferase and geranylgeranyltransferase inhibitor L‑778,123 and radiotherapy for locally advanced pancreatic cancer. Clin Cancer Res 10(16):5447–5454
Hahn SM et al (2002) A Phase I trial of the farnesyltransferase inhibitor L‑778,123 and radiotherapy for locally advanced lung and head and neck cancer. Clin Cancer Res 8(5):1065–1072
Sepp-Lorenzino L et al (1995) A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res 55(22):5302–5309
End DW et al (2001) Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res 61(1):131–137
Di Paolo A et al (2001) Inhibition of protein farnesylation enhances the chemotherapeutic efficacy of the novel geranylgeranyltransferase inhibitor BAL9611 in human colon cancer cells. Br J Cancer 84(11):1535–1543
Song SY et al (2000) K‑Ras-independent effects of the farnesyl transferase inhibitor L‑744,832 on cyclin B1/Cdc2 kinase activity, G2/M cell cycle progression and apoptosis in human pancreatic ductal adenocarcinoma cell. Neoplasia 2(3):261–272
Ross SJ et al (2017) Targeting KRAS-dependent tumors with AZD4785, a high-affinity therapeutic antisense oligonucleotide inhibitor of KRAS. Sci Transl Med 9(394):eaal5253. https://doi.org/10.1126/scitranslmed.aal5253
Aoki K et al (1995) Liposome-mediated in vivo gene transfer of antisense K‑ras construct inhibits pancreatic tumor dissemination in the murine peritoneal cavity. Cancer Res 55(17):3810–3816
Tsuchida T et al (1998) Hammerhead ribozyme specifically inhibits mutant K‑ras mRNA of human pancreatic cancer cells. Biochem Biophys Res Commun 253(2):368–373
Kita K et al (1999) Growth inhibition of human pancreatic cancer cell lines by anti-sense oligonucleotides specific to mutated K‑ras genes. Int J Cancer 80(4):553–558
Smakman N et al (2005) Dual effect of Kras(D12) knockdown on tumorigenesis: increased immune-mediated tumor clearance and abrogation of tumor malignancy. Oncogene 24(56):8338–8342
Golan T et al (2015) RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 6(27):24560–24570
Gort E et al (2020) A phase I, open-label, dose-escalation trial of BI 1701963 as monotherapy and in combination with trametinib in patients with KRAS mutated advanced or metastatic solid tumors. J Clin Oncol 38(15):TPS3651–TPS3651
Wee S et al (2009) PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res 69(10):4286–4293
Toulany M et al (2016) Dual targeting of PI3K and MEK enhances the radiation response of K‑RAS mutated non-small cell lung cancer. Oncotarget 7(28):43746–43761
Toulany M et al (2014) ERK2-dependent reactivation of Akt mediates the limited response of tumor cells with constitutive K‑RAS activity to PI3K inhibition. Cancer Biol Ther 15(3):317–328
Shapiro GI et al (2020) Phase Ib study of the MEK inhibitor cobimetinib (GDC-0973) in combination with the PI3K inhibitor pictilisib (GDC-0941) in patients with advanced solid tumors. Invest New Drugs 38(2):419–432
Lanman BA et al (2020) Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J Med Chem 63(1):52–65
Tanaka N et al (2021) Clinical acquired resistance to KRAS(G12C) inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discov 11(8):1913–1922
Awad MM et al (2021) Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med 384(25):2382–2393
Blaquier JB, Cardona AF, Recondo G (2021) Resistance to KRAS(G12C) inhibitors in non-small cell lung cancer. Front Oncol 11:787585
Mao Z et al (2022) KRAS(G12D) can be targeted by potent inhibitors via formation of salt bridge. Cell Discov 8(1):5
Hallin J et al (2022) Anti-tumor efficacy of a potent and selective non-covalent KRAS(G12D) inhibitor. Nat Med 28(10):2171–2182
Koltun E et al (2021) Abstract 1260: first-in-class, orally bioavailable KRASG12V(ON) tri-complex inhibitors, as single agents and in combinations, drive profound anti-tumor activity in preclinical models of KRASG12V mutant cancers. Cancer Res 81(13):1260–1260
Lasham A et al (2012) YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem J 449(1):11–23
Sangermano F, Delicato A, Calabrò V (2020) Y box binding protein 1 (YB-1) oncoprotein at the hub of DNA proliferation, damage and cancer progression. Biochimie 179:205–216
Serra V et al (2013) RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. J Clin Invest 123(6):2551–2563
Maier E et al (2019) Dual targeting of Y‑box binding protein‑1 and Akt inhibits proliferation and enhances the chemosensitivity of colorectal cancer cells. Cancers (Basel) 11(4):562. https://doi.org/10.3390/cancers11040562
Kosnopfel C et al (2017) Human melanoma cells resistant to MAPK inhibitors can be effectively targeted by inhibition of the p90 ribosomal S6 kinase. Oncotarget 8(22):35761–35775
Ushijima M et al (2022) An oral first-in-class small molecule RSK inhibitor suppresses AR variants and tumor growth in prostate cancer. Cancer Sci 113(5):1731–1738
Shibata T et al (2020) Targeting phosphorylation of Y‑box–binding protein YBX1 by TAS0612 and everolimus in overcoming antiestrogen resistance. Mol Cancer Ther 19(3):882–894
Tang KJ et al (2016) Focal adhesion kinase regulates the DNA damage response and its inhibition radiosensitizes mutant KRAS lung cancer. Clin Cancer Res 22(23):5851–5863
Dong S et al (2022) Ceritinib is a novel triple negative breast cancer therapeutic agent. Mol Cancer 21(1):138
Khatri A et al (2019) ABL kinase inhibition sensitizes primary lung adenocarcinomas to chemotherapy by promoting tumor cell differentiation. Oncotarget 10(20):1874–1886
Gupta K et al (2022) Identification of synergistic drug combinations to target KRAS-driven chemoradioresistant cancers utilizing tumoroid models of colorectal adenocarcinoma and recurrent glioblastoma. Front Oncol 12:840241
Khan N et al (2013) Fisetin: a dietary antioxidant for health promotion. Antioxidants Redox Signal 19(2):151–162
Syed DN et al (2008) Dietary agents for chemoprevention of prostate cancer. Cancer Lett 265(2):167–176
Sechi M et al (2018) Fisetin targets YB-1/RSK axis independent of its effect on ERK signaling: insights from in vitro and in vivo melanoma models. Sci Rep 8(1):15726
Khan MI et al (2014) YB‑1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget 5(9):2462–2474
Huang C et al (2022) ZC3H13-mediated N6-methyladenosine modification of PHF10 is impaired by fisetin which inhibits the DNA damage response in pancreatic cancer. Cancer Lett 530:16–28
Lin Y et al (2008) Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 8(7):634–646
Reipas KM et al (2013) Luteolin is a novel p90 ribosomal S6 kinase (RSK) inhibitor that suppresses Notch4 signaling by blocking the activation of Y‑box binding protein‑1 (YB-1). Oncotarget 4(2):329–345. https://doi.org/10.18632/oncotarget.834
Tanaka T et al (2021) 7‑Hydorxyindirubin is capable of specifically inhibiting anticancer drug-induced YB‑1 nuclear translocation without showing cytotoxicity in HepG2 hepatocellular carcinoma cells. Biochem Biophys Res Commun 544:15–21
Ma J‑W et al (2016) Aloe-emodin inhibits HER‑2 expression through the downregulation of Y‑box binding protein‑1 in HER-2-overexpressing human breast cancer cells. Oncotarget 7(37):58915–58930
Chan C et al (2016) Qualitative and quantitative analysis of chemical constituents of centipeda minima by HPLC-QTOF-MS & HPLC-DAD. J Pharm Biomed Anal 125:400–407
Liu YQ et al (2015) Skp1 in lung cancer: clinical significance and therapeutic efficacy of its small molecule inhibitors. Oncotarget 6(33):34953–34967
Li C et al (2018) Sesquiterpene lactone 6‑O-angeloylplenolin reverses vincristine resistance by inhibiting YB‑1 nuclear translocation in colon carcinoma cells. Oncol Lett 15(6):9673–9680
El Hage K et al (2023) Targeting RNA:protein interactions with an integrative approach leads to the identification of potent YBX1 inhibitors. Elife 12:e80387
Tailor D et al (2021) Y box binding protein 1 inhibition as a targeted therapy for ovarian cancer. Cell Chem Biol 28(8):1206–1220.e6
Gunasekaran VP et al (2018) Identification of 2,4-dihydroxy-5-pyrimidinyl imidothiocarbomate as a novel inhibitor to Y box binding protein‑1 (YB-1) and its therapeutic actions against breast cancer. Eur J Pharm Sci 116:2–14
Higashi K et al (2011) A novel small compound that promotes nuclear translocation of YB‑1 ameliorates experimental hepatic fibrosis in mice. J Biol Chem 286(6):4485–4492
Law JH et al (2010) Molecular decoy to the Y‑box binding protein‑1 suppresses the growth of breast and prostate cancer cells whilst sparing normal cell viability. PLoS One 5(9):e12661
Izumi H et al (2016) Optimal sequence of antisense DNA to silence YB‑1 in lung cancer by use of a novel polysaccharide drug delivery system. Int J Oncol 48(6):2472–2478
Funding
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, TO 685/2-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Khozooei, S. Veerappan and M. Toulany declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
S. Khozooei and S. Veerappan share first authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khozooei, S., Veerappan, S. & Toulany, M. YB-1 activating cascades as potential targets in KRAS-mutated tumors. Strahlenther Onkol 199, 1110–1127 (2023). https://doi.org/10.1007/s00066-023-02092-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02092-8