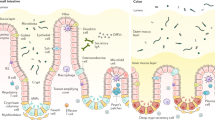
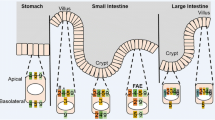
Abstract
Toll-like receptors (TLRs) are membrane-bound microbial sensors that mediate important host-to-microbe responses. Cell biology aspects of TLR function have been intensively studied in professional immune cells, in particular the macrophages and dendritic cells, but not well explored in other specialized epithelial cell types. The adult intestinal epithelial cells are in close contact with trillions of enteric microbes and engage in lifelong immune surveillance. Mature intestinal epithelial cells, in contrast to immune cells, are highly polarized. Recent studies suggest that distinct mechanisms may govern TLR traffic and compartmentalization in these specialized epithelial cells to establish and maintain precise signaling of individual TLRs. We, using immune cells as references, discuss here the shared and/or unique molecular machineries used by intestinal epithelial cells to control TLR transport, localization, processing, activation, and signaling. A better understanding of these mechanisms will certainly generate important insights into both the mechanism and potential intervention of leading digestive disorders, in particular inflammatory bowel diseases.


Similar content being viewed by others
References
Koropatkin NM, Cameron EA, Martens EC (2012) How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10(5):323–335
Kashyap PC et al (2013) Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci USA 110(42):17059–17064
Erkosar B et al (2013) Host-intestinal microbiota mutualism: “learning on the fly”. Cell Host Microbe 13(1):8–14
Pickard JM et al (2014) Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness. Nature 514(7524):638–641
Brestoff JR, Artis D (2013) Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 14(7):676–684
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157(1):121–141
Buffie CG, Pamer EG (2013) Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13(11):790–801
Maier L et al (2013) Microbiota-derived hydrogen fuels Salmonella typhimurium invasion of the gut ecosystem. Cell Host Microbe 14(6):641–651
Clemente JC et al (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270
Sekirov I et al (2010) Gut microbiota in health and disease. Physiol Rev 90(3):859–904
Crosnier C, Stamataki D, Lewis J (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7(5):349–359
Barker N (2014) Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15(1):19–33
Mabbott NA et al (2013) Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6(4):666–677
Wells JM et al (2011) Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA 108(Suppl 1):4607–4614
Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10(2):131–144
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14(3):141–153
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34(5):637–650
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7):499–511
Kang JY et al (2009) Recognition of lipopeptide patterns by toll-like receptor 2-toll-like receptor 6 heterodimer. Immunity 31(6):873–884
Jin MS et al (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130(6):1071–1082
Koblansky AA et al (2013) Recognition of profilin by toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38(1):119–130
Oldenburg M et al (2012) TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 337(6098):1111–1115
Rifkin IR et al (2005) Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev 204:27–42
Rakoff-Nahoum S, Medzhitov R (2009) Toll-like receptors and cancer. Nat Rev Cancer 9(1):57–63
Zhang P et al (2009) Cutting edge: cardiac myosin activates innate immune responses through TLRs. J Immunol 183(1):27–31
Barton GM, Kagan JC (2009) A cell biological view of toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol 9(8):535–542
Gay NJ et al (2014) Assembly and localization of toll-like receptor signalling complexes. Nat Rev Immunol 14(8):546–558
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 11(5):373–384
O’Neill LA, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in toll-like receptor signalling. Nat Rev Immunol 7(5):353–364
Blasius AL, Beutler B (2010) Intracellular toll-like receptors. Immunity 32(3):305–315
Otte JM, Cario E, Podolsky DK (2004) Mechanisms of cross hyporesponsiveness to toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126(4):1054–1070
Cario E et al (2002) Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol 160(1):165–173
Melmed G et al (2003) Human intestinal epithelial cells are broadly unresponsive to toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol 170(3):1406–1415
Lavelle EC et al (2010) The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol 3(1):17–28
Fusunyan RD et al (2001) Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res 49(4):589–593
Chabot S et al (2006) TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol 176(7):4275–4283
Cario E, Podolsky DK (2000) Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 68(12):7010–7017
Ortega-Cava CF et al (2003) Strategic compartmentalization of toll-like receptor 4 in the mouse gut. J Immunol 170(8):3977–3985
Hornef MW et al (2002) Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med 195(5):559–570
Ortega-Cava CF et al (2006) Epithelial toll-like receptor 5 is constitutively localized in the mouse cecum and exhibits distinctive down-regulation during experimental colitis. Clin Vaccine Immunol 13(1):132–138
Gewirtz AT et al (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167(4):1882–1885
Rhee SH et al (2005) Pathophysiological role of toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA 102(38):13610–13615
Bambou JC et al (2004) In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J Biol Chem 279(41):42984–42992
Barton GM, Kagan JC, Medzhitov R (2006) Intracellular localization of toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol 7(1):49–56
Onji M et al (2013) An essential role for the N-terminal fragment of toll-like receptor 9 in DNA sensing. Nat Commun 4:1949
Ewald SE et al (2008) The ectodomain of toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456(7222):658–662
Park B et al (2008) Proteolytic cleavage in an endolysosomal compartment is required for activation of toll-like receptor 9. Nat Immunol 9(12):1407–1414
Ewald SE et al (2011) Nucleic acid recognition by toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med 208(4):643–651
Mouchess ML et al (2011) Transmembrane mutations in toll-like receptor 9 bypass the requirement for ectodomain proteolysis and induce fatal inflammation. Immunity 35(5):721–732
Lee J et al (2006) Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8(12):1327–1336
Yu S et al (2014) TLR sorting by Rab11 endosomes maintains intestinal epithelial-microbial homeostasis. EMBO J 33(17):1882–1895
Rumio C et al (2004) Degranulation of Paneth cells via toll-like receptor 9. Am J Pathol 165(2):373–381
Rumio C et al (2012) Induction of Paneth cell degranulation by orally administered toll-like receptor ligands. J Cell Physiol 227(3):1107–1113
Schulz O, Pabst O (2013) Antigen sampling in the small intestine. Trends Immunol 34(4):155–161
De Matteis MA, Luini A (2008) Exiting the Golgi complex. Nat Rev Mol Cell Biol 9(4):273–284
Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10(8):513–525
Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91(1):119–149
Yu IM, Hughson FM (2010) Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol 26:137–156
Takahashi K et al (2007) A protein associated with toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med 204(12):2963–2976
Liu B et al (2010) Folding of toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun 1:79
Yang Y et al (2007) Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26(2):215–226
Bonifacino JS (2014) Adaptor proteins involved in polarized sorting. J Cell Biol 204(1):7–17
Grant BD, Donaldson JG (2009) Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10(9):597–608
Hsu VW, Bai M, Li J (2012) Getting active: protein sorting in endocytic recycling. Nat Rev Mol Cell Biol 13(5):323–328
Hirokawa N et al (2009) Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10(10):682–696
Roberts AJ et al (2013) Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol 14(11):713–726
Hammer JA 3rd, Sellers JR (2012) Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol 13(1):13–26
Verhey KJ, Hammond JW (2009) Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol 10(11):765–777
Seabra MC, Coudrier E (2004) Rab GTPases and myosin motors in organelle motility. Traffic 5(6):393–399
Hancock WO (2014) Bidirectional cargo transport: moving beyond tug of war. Nat Rev Mol Cell Biol 15(9):615–628
Ivanov II et al (2008) Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4(4):337–349
Martin-Belmonte F et al (2007) PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128(2):383–397
Rodriguez-Boulan E, Macara IG (2014) Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15(4):225–242
Leifer CA et al (2006) Cytoplasmic targeting motifs control localization of toll-like receptor 9. J Biol Chem 281(46):35585–35592
Lee BL et al (2013) UNC93B1 mediates differential trafficking of endosomal TLRs. Elife 2:e00291
Ohno H (2006) Clathrin-associated adaptor protein complexes. J Cell Sci 119(Pt 18):3719–3721
Hase K et al (2013) AP-1B-mediated protein sorting regulates polarity and proliferation of intestinal epithelial cells in mice. Gastroenterology 145(3):625–635
Takahashi D et al (2011) The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology 141(2):621–632
Shafaq-Zadah M et al (2012) AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 139(11):2061–2070
Setta-Kaffetzi N et al (2014) AP1S3 Mutations are associated with pustular psoriasis and impaired toll-like receptor 3 trafficking. Am J Hum Genet 94(5):790–797
Mantegazza AR et al (2012) Adaptor protein-3 in dendritic cells facilitates phagosomal toll-like receptor signaling and antigen presentation to CD4+ T cells. Immunity 36(5):782–794
Sasai M, Linehan MM, Iwasaki A (2010) Bifurcation of toll-like receptor 9 signaling by adaptor protein 3. Science 329(5998):1530–1534
Blasius AL et al (2010) Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA 107(46):19973–19978
Weisz OA, Rodriguez-Boulan E (2009) Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci 122(Pt 23):4253–4266
Weber AN, Morse MA, Gay NJ (2004) Four N linked glycosylation sites in human toll-like receptor 2 cooperate to direct efficient biosynthesis and secretion. J Biol Chem 279(33):34589–34594
Sun J et al (2006) Structural and functional analyses of the human toll-like receptor 3. Role of glycosylation. J Biol Chem 281(16):11144–11151
Istomin AY, Godzik A (2009) Understanding diversity of human innate immunity receptors: analysis of surface features of leucine-rich repeat domains in NLRs and TLRs. BMC Immunol 10:48
Xu S et al (2011) A Rab11a-enriched subapical membrane compartment regulates a cytoskeleton-dependent transcytotic pathway in secretory epithelial cells of the lacrimal gland. J Cell Sci 124(Pt 20):3503–3514
Roland JT et al (2011) Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci USA 108(7):2789–2794
Husebye H et al (2010) The Rab11a GTPase controls toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity 33(4):583–596
Wang D et al (2010) Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc Natl Acad Sci USA 107(31):13806–13811
Schuck S et al (2007) Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic 8(1):47–60
Chen S et al (2014) SEC-10 and RAB-10 coordinate basolateral recycling of clathrin-independent cargo through endosomal tubules in Caenorhabditis elegans. Proc Natl Acad Sci USA 111(43):15432–15437
Tuma P, Hubbard AL (2003) Transcytosis: crossing cellular barriers. Physiol Rev 83(3):871–932
Su T et al (2010) A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol 12(12):1143–1153
Piper RC, Luzio JP (2007) Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol 19(4):459–465
Chiang CY et al (2012) Cofactors required for TLR7- and TLR9-dependent innate immune responses. Cell Host Microbe 11(3):306–318
Husebye H et al (2006) Endocytic pathways regulate toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J 25(4):683–692
Mashukova A, Wald FA, Salas PJ (2011) Tumor necrosis factor alpha and inflammation disrupt the polarity complex in intestinal epithelial cells by a posttranslational mechanism. Mol Cell Biol 31(4):756–765
St Johnston D, Ahringer J (2010) Cell polarity in eggs and epithelia: parallels and diversity. Cell 141(5):757–774
Pasparakis M (2009) Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol 9(11):778–788
Eyster KM (2007) The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ 31(1):5–16
Kagan JC, Medzhitov R (2006) Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell 125(5):943–955
Balla T (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev 93(3):1019–1137
Tabeta K et al (2006) The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via toll-like receptors 3, 7 and 9. Nat Immunol 7(2):156–164
Lee BL, Barton GM (2014) Trafficking of endosomal toll-like receptors. Trends Cell Biol 24(6):360–369
Brinkmann MM et al (2007) The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol 177(2):265–275
Kim J et al (2013) Acidic amino acid residues in the juxtamembrane region of the nucleotide-sensing TLRs are important for UNC93B1 binding and signaling. J Immunol 190(10):5287–5295
Itoh H et al (2011) UNC93B1 physically associates with human TLR8 and regulates TLR8-mediated signaling. PLoS One 6(12):e28500
Funami K et al (2004) The cytoplasmic ‘linker region’ in toll-like receptor 3 controls receptor localization and signaling. Int Immunol 16(8):1143–1154
Pohar J et al (2012) The role of UNC93B1 protein in surface localization of TLR3 receptor and in cell priming to nucleic acid agonists. J Biol Chem 288(1):442–454
Huh JW et al (2014) UNC93B1 is essential for the plasma membrane localization and signaling of toll-like receptor 5. Proc Natl Acad Sci USA 111(19):7072–7077
Qi R, Singh D, Kao CC (2012) Proteolytic processing regulates toll-like receptor 3 stability and endosomal localization. J Biol Chem 287(39):32617–32629
Pelka K et al (2014) Cutting edge: the UNC93B1 tyrosine-based motif regulates trafficking and TLR responses via separate mechanisms. J Immunol 193(7):3257–3261
Latz E et al (2002) Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem 277(49):47834–47843
Ishihara S et al (2004) Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J Immunol 173(2):1406–1416
Lee CC, Avalos AM, Ploegh HL (2012) Accessory molecules for toll-like receptors and their function. Nat Rev Immunol 12(3):168–179
Zanoni I et al (2011) CD14 controls the LPS-induced endocytosis of toll-like receptor 4. Cell 147(4):868–880
Jiang Z et al (2005) CD14 is required for MyD88-independent LPS signaling. Nat Immunol 6(6):565–570
Abreu MT et al (2002) TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem 277(23):20431–20437
Frolova L et al (2008) Expression of toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem 56(3):267–274
Liaunardy-Jopeace A, Bryant CE, Gay NJ (2014) The COP II adaptor protein TMED7 is required to initiate and mediate the delivery of TLR4 to the plasma membrane. Sci Signal 7(336):ra70
Acknowledgments
This work was supported by the National Institute of Health (NIH) Grants DK085194, DK093809, DK102934, and CA178599; Charles and Johanna Busch Memorial Award (659160); NSF/BIO/IDBR (1353890) and Rutgers University Faculty Research Grant (281708). S.Y. was supported by New Jersey Commission on Cancer Research Postdoctoral Fellowship (DFHS13PPC016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, S., Gao, N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell. Mol. Life Sci. 72, 3343–3353 (2015). https://doi.org/10.1007/s00018-015-1931-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1931-1