Abstract
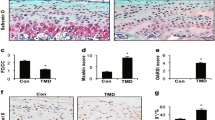
Discoidin domain receptor 1 (DDR-1)-deficient mice exhibited a high incidence of osteoarthritis (OA) in the temporomandibular joint (TMJ) as early as 9 weeks of age. They showed typical histological signs of OA, including surface fissures, loss of proteoglycans, chondrocyte cluster formation, collagen type I upregulation, and atypical collagen fibril arrangements. Chondrocytes isolated from the TMJs of DDR-1-deficient mice maintained their osteoarthritic characteristics when placed in culture. They expressed high levels of runx-2 and collagen type I, as well as low levels of sox-9 and aggrecan. The expression of DDR-2, a key factor in OA, was increased. DDR-1-deficient chondrocytes from the TMJ were positively influenced towards chondrogenesis by a three-dimensional matrix combined with a runx-2 knockdown or stimulation with extracellular matrix components, such as nidogen-2. Therefore, the DDR-1 knock-out mouse can serve as a novel model for temporomandibular disorders, such as OA of the TMJ, and will help to develop new treatment options, particularly those involving tissue regeneration.







Similar content being viewed by others
References
Wadhwa S, Embree M, Ameye L, Young MF (2005) Mice deficient in biglycan and fibromodulin as a model for temporomandibular joint osteoarthritis. Cells Tissues Organs 181(3–4):136–143. doi:10.1159/000091375
Carlson GE, Magnusson T (1999) Management of temporomandibular disorders in the general dental practice, vol 1. Quintessenz, Berlin
Lohmander LS, Roos EM (2007) Clinical update: treating osteoarthritis. Lancet 370(9605):2082–2084. doi:10.1016/s0140-6736(07)61879-0
Buckwalter JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect 47:487–504
Horton WE Jr, Bennion P, Yang L (2006) Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskel Neuronal Interact 6(4):379–381
Poole AR (1999) An introduction to the pathophysiology of osteoarthritis. Front Biosci 4:D662–D670
Sandell LJ (2007) Modern molecular analysis of a traditional disease: progression in osteoarthritis. Arthr Rheum 56(8):2474–2477. doi:10.1002/art.22760
Kruegel J, Sadowski B, Miosge N (2008) Nidogen-1 and nidogen-2 in healthy human cartilage and in late-stage osteoarthritis cartilage. Arthr Rheum 58(5):1422–1432. doi:10.1002/art.23480
Kuettner KE (1992) Biochemistry of articular cartilage in health and disease. Clin Biochem 25(3):155–163
Loeser RF (2000) Chondrocyte integrin expression and function. Biorheology 37(1–2):109–116
Goldring MB, Otero M (2011) Inflammation in osteoarthritis. Curr Opin Rheumatol 23(5):471–478. doi:10.1097/BOR.0b013e328349c2b1
Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N (2009) Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 4(4):324–335. doi:10.1016/j.stem.2009.01.015
Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, Walter MW, Lehman AD, Ding L, Buckwalter JA, Martin JA (2012) Chondrogenic progenitor cells respond to cartilage injury. Arthr Rheum 64(11):3626–3637. doi:10.1002/art.34613
Silbermann M, Livne E (1979) Age-related degenerative changes in the mouse mandibular joint. J Anat 129(Pt 3):507–520
Rintala M, Metsaranta M, Saamanen AM, Vuorio E, Ronning O (1997) Abnormal craniofacial growth and early mandibular osteoarthritis in mice harbouring a mutant type II collagen transgene. J Anat 190(Pt 2):201–208
Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, Li Y (2003) Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho). Arthr Rheum 48(9):2509–2518. doi:10.1002/art.11233
Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, Li Y (2011) Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol 179(3):1338–1346. doi:10.1016/j.ajpath.2011.05.023
Lam NP, Li Y, Waldman AB, Brussiau J, Lee PL, Olsen BR, Xu L (2007) Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Arch Oral Biol 52(6):579–584. doi:10.1016/j.archoralbio.2006.10.014
Vogel W (1999) Discoidin domain receptors: structural relations and functional implications. FASEB J 13(Suppl):S77–S82
Vogel W, Gish GD, Alves F, Pawson T (1997) The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1(1):13–23
Foehr ED, Tatavos A, Tanabe E, Raffioni S, Goetz S, Dimarco E, De Luca M, Bradshaw RA (2000) Discoidin domain receptor 1 (DDR1) signaling in PC12 cells: activation of juxtamembrane domains in PDGFR/DDR/TrkA chimeric receptors. FASEB J 14(7):973–981
Vogel WF, Aszodi A, Alves F, Pawson T (2001) Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol 21(8):2906–2917. doi:10.1128/MCB.21.8.2906-2917.2001
Little C, Smith S, Ghosh P, Bellenger C (1997) Histomorphological and immunohistochemical evaluation of joint changes in a model of osteoarthritis induced by lateral meniscectomy in sheep. J Rheumatol 24(11):2199–2209
Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg A, Heinegard D (1992) Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem 267(9):6132–6136
Koelling S, Clauditz TS, Kaste M, Miosge N (2006) Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthr Res Ther 8(3):R56. doi:10.1186/ar1922
Gersdorff N, Kohfeldt E, Sasaki T, Timpl R, Miosge N (2005) Laminin gamma 3 chain binds to nidogen and is located in murine basement membranes. J Biol Chem 280(23):22146–22153. doi:10.1074/jbc.M501875200
Hauselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, Kuettner KE, Thonar EJ (1994) Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci 107(Pt 1):17–27
Diaz-Romero J, Gaillard JP, Grogan SP, Nesic D, Trub T, Mainil-Varlet P (2005) Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol 202(3):731–742. doi:10.1002/jcp.20164
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Goldring MB, Goldring SR (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann NY Acad Sci 1192:230–237. doi:10.1111/j.1749-6632.2009.05240.x
Kvist AJ, Nystrom A, Hultenby K, Sasaki T, Talts JF, Aspberg A (2008) The major basement membrane components localize to the chondrocyte pericellular matrix—a cartilage basement membrane equivalent? Matrix Biol 27(1):22–33. doi:10.1016/j.matbio.2007.07.007
Holtzer H, Abbott J, Lash J, Holtzer S (1960) The loss of phenotypic traits by differentiated cells in vitro, I. Dedifferentiation of cartilage cells. Proc Natl Acad Sci USA 46(12):1533–1542
Ruiz PA, Jarai G (2011) Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J Biol Chem 286(15):12912–12923. doi:10.1074/jbc.M110.143693
Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ (2006) Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet 2(12):e216. doi:10.1371/journal.pgen.0020216
Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, Hsu C, Ali SA, Alman BA (2009) Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med 15(12):1421–1425. doi:10.1038/nm.2055
Enomoto H, Inoki I, Komiya K, Shiomi T, Ikeda E, Obata K, Matsumoto H, Toyama Y, Okada Y (2003) Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am J Pathol 162(1):171–181
Reich A, Maziel SS, Ashkenazi Z, Ornan EM (2010) Involvement of matrix metalloproteinases in the growth plate response to physiological mechanical load. J Appl Physiol 108(1):172–180. doi:10.1152/japplphysiol.00821.2009
Whitfield JF (2008) The solitary (primary) cilium—a mechanosensory toggle switch in bone and cartilage cells. Cell Signal 20(6):1019–1024. doi:10.1016/j.cellsig.2007.12.001
Martinez-Sanchez A, Dudek KA, Murphy CL (2012) Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145). J Biol Chem 287(2):916–924. doi:10.1074/jbc.M111.302430
Mattijssen S, Welting TJ, Pruijn GJ (2010) RNase MRP and disease. Wiley Interdiscip Rev RNA 1(1):102–116. doi:10.1002/wrna.9
Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H (2006) Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthr Rheum 54(8):2462–2470. doi:10.1002/art.22041
Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y (2005) Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem 280(1):548–555. doi:10.1074/jbc.M411036200
Otero M, Plumb DA, Tsuchimochi K, Dragomir CL, Hashimoto K, Peng H, Olivotto E, Bevilacqua M, Tan L, Yang Z, Zhan Y, Oettgen P, Li Y, Marcu KB, Goldring MB (2012) E74-like factor 3 (ELF3) impacts on matrix metalloproteinase 13 (MMP13) transcriptional control in articular chondrocytes under proinflammatory stress. J Biol Chem 287(5):3559–3572. doi:10.1074/jbc.M111.265744
Sunk IG, Bobacz K, Hofstaetter JG, Amoyo L, Soleiman A, Smolen J, Xu L, Li Y (2007) Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthr Rheum 56(11):3685–3692. doi:10.1002/art.22970
Wann AK, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, Knight MM (2012) Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J 26(4):1663–1671. doi:10.1096/fj.11-193649
Shao YY, Wang L, Welter JF, Ballock RT (2012) Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone 50(1):79–84. doi:10.1016/j.bone.2011.08.033
Chang CF, Ramaswamy G, Serra R (2012) Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthr Cartil 20(2):152–161. doi:10.1016/j.joca.2011.11.009
Symons NB (1965) A histochemical study of the secondary cartilage of the mandibular condyle in the rat. Arch Oral Biol 10(4):579–584
Shen G, Darendeliler MA (2005) The adaptive remodeling of condylar cartilage—a transition from chondrogenesis to osteogenesis. J Dent Res 84(8):691–699
Benjamin M, Ralphs JR (2004) Biology of fibrocartilage cells. Int Rev Cytol 233:1–45. doi:10.1016/s0074-7696(04)33001-9
Koelling S, Miosge N (2010) Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthr Rheum 62(4):1077–1087. doi:10.1002/art.27311
Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B (1999) Sox9 is required for cartilage formation. Nat Genet 22(1):85–89. doi:10.1038/8792
Tallheden T, Karlsson C, Brunner A, Van Der Lee J, Hagg R, Tommasini R, Lindahl A (2004) Gene expression during redifferentiation of human articular chondrocytes. Osteoarthr Cartil 12(7):525–535. doi:10.1016/j.joca.2004.03.004
Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y (2007) Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthr Rheum 56(8):2663–2673. doi:10.1002/art.22761
Acknowledgments
The authors would like to thank the staff of the animal facilities at the MPI Experimental Medicine, Goettingen and the Medical Faculty of the University of Goettingen for animal care, Dr. Bunt, Molecular & Live Cell Imaging (MOLCI), central imaging facility of the UMG, for the confocal microscopy work, Dr. Salinas-Riester for performing the microarray and Mr. Opitz for statistical evaluation, and Dr. Dullin for help with the micro-CT. We would also like to thank Mr. Hoehne, as parts of the results were taken from his doctoral thesis. We also wish to thank Mr. Menrath for professional assistance with the figure layout.
Author information
Authors and Affiliations
Corresponding authors
Additional information
B. Schminke and H. Muhammad contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.

18_2013_1436_Fig8_ESM.jpg
Supplemental Fig. 1 The role of runx-2 as a transcription factor in the osteoblastic lineage. a DAPI staining of a chondrocytes nucleus, b immunocytochemistry of runx-2, localization in the cytoplasm and nucleus of the same chondrocyte was observed. c Merged image of a and b for better visualization of runx-2 in the nucleus. Overexpression of runx-2. Lane 1 control with the vector without insert; lane 2 page ruler; and lane 3 overexpressed runx-2. Coomassie blue staining was performed to determine the overall protein pattern and to confirm equal loading, as observed in d. Western blot of runx-2, as observed in e. Measured mRNA expression levels of osteogenic markers of the cells after overexpression of runx-2. We observed enhanced gene expression of f runx-2, g Col1A1, h SPP1 and i IBSP. *Significant differences (p ≤ 0.05); data are mean values with SD from three individual experiments (JPEG 2551 kb)

18_2013_1436_Fig9_ESM.jpg
Supplemental Fig. 2 Preliminary investigation of primary cilia in TMJ chondrocytes. a Microarray results of genes associated with primary cilia. b DAPI staining (left) of the nuclei of the chondrocytes and acetylated α-tubulin staining (red for better visualization) of the primary cilia (right) are shown. c The quantification of the results indicated a tendency towards a reduction of the percentage of cells with primary cilia in DDR-1 KO chondrocytes. Data are mean values with SD of three individual experiments. (n = 6, including three KO mice and three WT mice, for cilia staining) (JPEG 5676 kb)
Rights and permissions
About this article
Cite this article
Schminke, B., Muhammad, H., Bode, C. et al. A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell. Mol. Life Sci. 71, 1081–1096 (2014). https://doi.org/10.1007/s00018-013-1436-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1436-8